INTRODUCTION
When doing parasitological research in the field, species with infrequent occurrence and/or low intensity are routinely observed (Bush and Holmes, Reference Bush and Holmes1986; Norton et al. Reference Norton, Lewis and Rollinson2003; Poulin, Reference Poulin2007a ; Kennedy, Reference Kennedy2012). While such rarely found species are noticed only when large outbreaks occur (e.g. Pennella sp. from the Pacific saury Cololabis saira, Nagasawa et al. Reference Nagasawa, Imai and Ishida1988), patterns of spatial distribution and population dynamics have been poorly understood, as the trait of rarity itself makes quantitative sampling difficult. Moreover, the rare occurrences are often mixed up with accidental infections and/or sampling noise without much consideration (Bush et al. Reference Bush, Fernández, Esch and Seed2001). In this context, Bush et al. (Reference Bush, Fernández, Esch and Seed2001) provided an empathetic comment: ‘There are many parasites that are rare (using any connotation of the word; just why they are rare, and how they circumvent extinction, would be an interesting avenue to explore!)’
Species with a low abundance and/or narrow habitat-use range (Gaston, Reference Gaston, Kunin and Gaston1997), are at a disadvantage in avoiding extinction (see Gaston, Reference Gaston1994; Mace and Kershaw, Reference Mace, Kershaw, Kunin and Gaston1997). If a parasite has a narrow range of geographic distribution and/or host use, it risks being trapped by climate change and/or anthropogenic/catastrophic disturbances causing loss of prime habitat (Bush and Kennedy, Reference Bush and Kennedy1994; Poulin and Morand, Reference Poulin and Morand2004). Small populations risk disappearing simply through demographic and environmental stochastic fluctuations (Menges, Reference Menges, Fiedler and Jain1992) and genetic deterioration (Karron, Reference Karron, Kunin and Gaston1997). Furthermore, if a parasite prefers a specific environment, its local populations are likely to be isolated from each other with increasing the risk of extinction (Price, Reference Price1980; Rózsa, Reference Rózsa1992; Poulin and Morand, Reference Poulin and Morand2004). Nevertheless, rarely found parasites are occupying a particular niche in the given ecosystem to survive, reproduce and pass their genes onto future generations.
To compensate for the risk of extinction, specific population features are expected for rarely found parasites insuring the exploitation of targeted hosts. While asexual species have a chance to persist even with small local populations by self-reproduction (Ogawa et al. Reference Ogawa, Shirakashi and Ishitani2014), sexual species require to find mating partners (Kennedy, Reference Kennedy and Kennedy1976). Therefore, sexually reproducing parasites might form spatiotemporal hot spots of infection, thereby finding mating partners and acting as sources of parasite populations to colonize to other patches (Hartvigsen and Halvorsen, Reference Hartvigsen and Halvorsen1993, Reference Hartvigsen and Halvorsen1994; see also Bush and Kennedy, Reference Bush and Kennedy1994). Extinction risk will be also reduced by dispersal among local populations, via rescue effects or metapopulation processes (Levins, Reference Levins1969; Hanski, Reference Hanski1999). Therefore, spatial population structure and dispersal ability are fundamental to understanding survival strategies in rarely found parasites.
In this study, we conducted an intensive epidemiological survey of a piscicolid leech Taimenobdella amurensis, narrowly distributed in the Far East (Epshtein, Reference Epshtein1964; Lukin, Reference Lukin1976; Furness et al. Reference Furness, Williams, Nagasawa and Burreson2007), to elucidate spatial population structure and potential dispersal of this rare parasite. We also examined temporal changes of prevalence among local habitats by using 4-year data. When local dynamics are highly synchronous, considerable amount of dispersal among habitat patches is suggested, which results in a large panmictic population structure (Hanski, Reference Hanski1999; Koizumi, Reference Koizumi2011). Alternatively, when dynamics are completely independent, a set of isolated populations is suggested. Finally, when local dynamics are moderately synchronous, metapopulation structure with some dispersal among local habitats is suggested (Hanski, Reference Hanski1999; Koizumi, Reference Koizumi2011). We have some advantages in the study system to understand population structure and dynamics in rare parasites. First, without sacrificing hosts, each parasite can be easily distinguished in the field due to size and colour (Furness et al. Reference Furness, Williams, Nagasawa and Burreson2007). Second, biology of host fishes, including population dynamics and environmental characteristics, have been intensively studied more than 15 years (Koizumi, Reference Koizumi2011; Koizumi and Shimatani, Reference Koizumi and Shimatani2016), providing useful basic information.
MATERIALS AND METHODS
Study species and area
Taimenobdella amurensis is a fish leech found in the Amur basin and Hokkaido (northern Japan) in the Far East (Epshtein, Reference Epshtein1964; Lukin, Reference Lukin1976; Furness et al. Reference Furness, Williams, Nagasawa and Burreson2007). This sexually reproducing leech has been reported on various fishes (mainly salmonid fishes) (Furness et al. Reference Furness, Williams, Nagasawa and Burreson2007; Nagasawa et al. Reference Nagasawa, Kikuchi, Arakane and Yusa2009), but is known as a rare species possessing an extremely low prevalence and intensity (Nagasawa et al. Reference Nagasawa, Kikuchi, Arakane and Yusa2009). Only 16 cases of infection by this parasite were observed from 2001 to 2008 at the Chitose Salmon Aquarium (42°49′58″N, 141°39′33″E) neighbouring the middle-reach of the Chitose River, Hokkaido, Japan (Nagasawa et al. Reference Nagasawa, Kikuchi, Arakane and Yusa2009). Aquarium staffs keep daily records of leech infections on wild fishes via observation in a room equipped with acrylic underwater-viewing windows (ca. 7 windows with 1 m high × 2 m wide); however, only 30 individuals were recorded during the 7-year survey (Nagasawa et al. Reference Nagasawa, Kikuchi, Arakane and Yusa2009). As of yet, the population biology, including habitat and detailed host use, of this leech is still largely unknown.
Sampling was conducted in the upper Sorachi River system in central Hokkaido, Japan (Fig. 1), located upstream from a large reservoir (i.e. Kanayama reservoir; 43°10′12″N, 142°31′48″E). The riverine structure is characterized by more than 100 small tributaries (length, <500 m; base flow, c. 0·01–0·5 m3 s−1; width, c. 0·5–3·0 m; depth, c. 5–20 cm) directly connecting to the main stem channel (base flow, c. 1·0–10 m3 s−1; width, c. 5–30 m; depth, c. 50–100 cm) (see Koizumi and Maekawa, Reference Koizumi and Maekawa2003, Reference Koizumi and Maekawa2004; Koizumi et al. Reference Koizumi, Yamamoto and Maekawa2006a , Reference Koizumi, Yamamoto and Maekawa b ; Koizumi and Shimatani, Reference Koizumi and Shimatani2016). In addition to these small tributaries, two branches (i.e. the Nigorisawa Creek and the Shimizusawa Creek) diverge in the upper reaches of the main stem, named as the Shiisorapuchi River (Fig. 1). The substratum of most tributaries consists mainly of pebble and cobble gravels, and other compositions (i.e. sand and silt) can be found depending on the place and stream structure. The main stem of the river generally has a boulder bottom.

Fig. 1. Map of the river system. Diagrams in the map indicate sampling localities of a spring-fed tributary (circle), non-spring-fed tributary (triangle) and main stem (rectangle). Abbreviations represent site name for additional small-scale surveys.
These tributaries and creeks of the Shiisorapuchi River can be divided into spring-fed or non-spring-fed types, based on the presence or absence of spring flow that affects water temperature and water discharge variations. Spring-fed tributaries have stable temperatures (5–8 °C) and water discharge throughout the year (Koizumi and Maekawa, Reference Koizumi and Maekawa2003; I. Koizumi, unpublished data). The other type of streams, i.e. non-spring-fed tributaries, are fed by rain and snowmelt runoff, precipitation and shallow ground water; thus, their water temperatures and discharge easily fluctuate on a daily and seasonal basis, ranging from 0 to 16 °C (Koizumi and Maekawa, Reference Koizumi and Maekawa2003; I. Koizumi, unpublished data).
Eleven fish species have been reported in the upper Sorachi River system (Koizumi and Maekawa, Reference Koizumi and Maekawa2003; Koizumi et al. Reference Koizumi, Hasegawa and Kishi2012) including far eastern brook lamprey Lethenteron sp. (northern form), Rosyface dace Tribolodon sachalinensis, Japanese dace Tribolodon hakonensis, Siberian stone loach Nemachelis barbatulus toni, Hokkaido eight-barbel loach Lefua costata nikkonis, Sakhalin taimen Parahucho perryi, rainbow trout Onchorhynchus mykiss, white-spotted char Salvelinus leucomaenis leucomaenis, Dolly Varden, Salvelinus malma krascheninnikovi, nine-spined stickleback Pungitius sp. (freshwater type) and freshwater sculpin Cottus nozawae. Only rainbow trout are a non-native species.
Each species of salmonid, believed to be main hosts (Furness et al. Reference Furness, Williams, Nagasawa and Burreson2007; Nagasawa et al. Reference Nagasawa, Kikuchi, Arakane and Yusa2009), has different distributional patterns and migratory tendencies, which should significantly influence the dynamics of the parasite leech. Dolly Varden occupy upper areas, especially spring-fed tributaries, whereas Sakhalin taimen occupies lower areas, including Kanayama reservoir (Edo et al. Reference Edo, Kawamula and Higashi2000; Koizumi et al. Reference Koizumi, Hasegawa and Kishi2012). White-spotted charr are in between but distribute most widely, from upper areas to the reservoir. Most Sakhalin taimen and some white-spotted charr migrate between the river and reservoir, probably moving several dozen kilometres (I. Koizumi, personal observation). They spawn in the main stem and large tributaries but not in small tributaries. Dolly Varden, on the other hand, migrate between small tributaries and the main stem, but not migrating to the reservoir (Koizumi et al. Reference Koizumi, Yamamoto and Maekawa2006a ; Ayer et al. Reference Ayer, Katahira, Fukui and Koizumi2017). They also spawn mostly in small tributaries but not in the main stem. Microsatellite DNA analysis indicates that dispersal of Dolly Varden may be several kilometres (Koizumi et al. Reference Koizumi, Yamamoto and Maekawa2006b ). This species forms a metapopulation structure; each tributary-based population is connected to other local populations with a moderate level of dispersal (Koizumi and Maekawa, Reference Koizumi and Maekawa2004; Koizumi et al. Reference Koizumi, Yamamoto and Maekawa2006b , Reference Koizumi, Yamamoto, Nomoto and Maekawa2008; Koizumi, Reference Koizumi2011). Non-native rainbow trout are much less abundant and life history is poorly known.
Field samplings
We conducted intensive sampling in spring after snowmelt (16–19 June 2013, 23 May and 15–19 June 2014, 20–27 June 2015, and 23–30 June 2016), which was a part of an annual population census of Dolly Varden (Koizumi et al. Reference Koizumi, Yamamoto, Nomoto and Maekawa2008; Koizumi, Reference Koizumi2011). To investigate seasonal changes of the parasite occurrence and annual life cycle, we conducted additional autumn sampling before snowfall (24–25 October 2013 and 4–5 September and 24–25 October 2014), although the sampling efforts (e.g. number of sites, sampling time) were much lower in autumn sampling. In total, 10 sites of the main stem and 28 tributaries were investigated to cover the entire area of the river system (Fig. 1); the tributaries consisted of 14 spring-fed and 14 non-spring-fed types. At these sites, we caught host fishes using a backpack electrofisher (Model 12B, Smith-Root, Vancouver, Canada). Sampling effort ranged from one to three screening passes with approximately 30–60 min per pass at each site. In addition to this basin-scale investigation, additional small-scale surveys were carried out (1) in a branch (i.e. Shimizusawa Creek) and tributaries (named as KU and SI) to examine the longitudinal distribution and (2) in a side channel of the main stem close to tributaries (i.e. KU, T10, T20, T49 and T50) to discriminate microscale differences in leech occurrence inside and outside the tributary (Fig. 1).
The fishes captured were anaesthetized by FA100 (DS Pharma Animal Health Co., Ltd., Osaka, Japan) in the field, and then measured for their total length or fork length (FL) to the nearest 1 mm and immediately checked for leech infection. All examined fishes were released into recovery sites after the procedure. For species identification, a portion of the leeches found were haphazardly collected and brought back to the laboratory of Hokkaido University, where they were relaxed by dripping 70% ethanol and subsequently refixed with 70–80% ethanol. The fixed specimens were measured for whole body length to the nearest 1 mm. Identification was based on the descriptions provided by Furness et al. (Reference Furness, Williams, Nagasawa and Burreson2007).
Statistical analyses
Infection indices were calculated using the definition provided by Bush et al. (Reference Bush, Lafferty, Lotz and Shostak1997).
Host use of T. amurensis in the present river system was evaluated with applying dietary-preference indices of Krebs (Reference Krebs1999) to the occurrence data: W i = O i /P i and B i = W i /ΣW, where W i is the infection ratio for host species i, O i is the proportion of infected host i individuals in the infected individuals of all host fishes, P i the is proportion of host i in all the examined host fishes and B i is the standardized value of W i . Fish with a W i value above 1·0 can be regarded as preferred hosts for the leech, while values of B i below or above ‘1/number of host species’ correspond to relative avoidance or preference (see Krebs, Reference Krebs1999).
To elucidate spatial occurrence patterns of leeches in the river system, generalized linear mixed models (GLMMs) with logit link function were fitted for the prevalence data (i.e. presence or absence of the leech on host individuals) obtained from each site. In this analysis, habitat type (main stem, spring-fed and non-spring-fed tributary) and geographical distance from the downstream end of the river were tested as explanatory variables, with the random effects of sampling site and sampling term taken into account. Geographical distances in each tributary were measured from the inlet of the Kanayama reservoir along with the river line with an open source map (Geospatial Information Authority of Japan; http://maps.gsi.go.jp) using Image J 1.46r. The candidate models were selected based on Akaike's information criterion (AIC; Burnham and Anderson, Reference Burnham and Anderson2002); the difference in AIC value (ΔAIC) between a constructed model and the best model with the lowest AIC value was checked for the model selections.
Occurrence surveys of rarely found parasites often produce unreliable data with many absent (i.e. zero-inflated situation), owing to miss examinations of host individuals distributed apart from infectious hot spots or improper timing of sampling when parasite infection rate is low (see Martin et al. Reference Martin, Wintle, Rhodes, Kuhnert, Field, Low-Choy, Tyre and Possingham2005; Zuur et al. Reference Zuur, Ieno, Walker, Saveliev and Smith2009). Such sampling biases should violate the general aggression pattern of macroparasites, which is represented by a negative binomial (NB) distribution model (Shaw and Dobson, Reference Shaw and Dobson1995; Poulin, Reference Poulin2013). Therefore, to investigate whether the probability distribution of the parasite infection follows a NB when considering only the infectious hot spots, model selections and comparisons were conducted based on the abundance data from the most preferred host in the prevalent sites. We compared three error distributions, i.e. NB, zero-inflated Poisson (ZIP) and zero-inflated negative binomial (ZINB) (Zuur et al. Reference Zuur, Ieno, Walker, Saveliev and Smith2009). Body size of the preferred host, sampling season and year were tested as candidates for explanatory variables in the fittings, with sampling site difference as a random effect. The best models of these probability distributions were subsequently compared with each other, according to the values of AIC.
Due to the seasonal gap in samplings (i.e. spring and autumn), it was also expected that body sizes of the leeches would differ between samplings. This difference was confirmed by the construction of a general linear model (LM) involving categorical explanatory variables of season and year. The leech specimens examined were haphazardly collected through the above-mentioned surveys. AIC was used for the selective criterion for the constructed models, as mentioned above.
We also assessed the level of synchrony in local dynamics to examine a possible population structure and connectivity. We calculated pairwise synchrony of the leech prevalence between the preferred patches (i.e. tributaries and the branch) using cross-correlation coefficient with no time lag (Bjørnstad et al. Reference Bjørnstad, Ims and Lambin1999; see also Koizumi et al. Reference Koizumi, Yamamoto, Nomoto and Maekawa2008). A 95% bootstrap confidence interval for the overall average synchrony was estimated by 10 000 permutations of sampling with replacements of the coefficients (Bjørnstad et al. Reference Bjørnstad, Ims and Lambin1999).
All statistical tests were performed using R 3.1.2 (R Development Core Team, 2014). Model fittings in the cases of binomial, NB, ZIP and ZINB error distributions employed the glmmADMB package (Fournier et al. Reference Fournier, Skaug, Ancheta, Ianelli, Magnusson, Maunder, Nielsen and Sibert2012).
RESULTS
Infection rate and host preference
In total, 1102 (5·3%) of 20 664 fish examined were infected with the leech during 2013–2016 (Table 1). The number of the leeches recovered was 304, 418, 420 and 206 individuals in the order of the year. Most of the individuals were collected from Dolly Varden (96·07%), while the other fishes, i.e. white-spotted charr (2·60%) and freshwater sculpins (1·26%), were also infected with the leech depending on the site.
Table 1. Prevalence of the fish leech Taimenobdella amurensis in host species and habitats. Bold indicates the habitats where the leeches were found and the preferred hosts provided as W and B (see details in the text)

a A few samples excluded due to measurement error.
Both host-preference indices, i.e. W and B, were always highest in Dolly Varden (Table 1). However, in some sites (uppermost reaches of the main stem and spring-fed tributaries) on the sampling of June 2013, for example, these indices for freshwater sculpin also showed relatively high value as a preferred host.
Leech distribution
The leeches occurred heterogeneously in the river system, confined to spring-fed tributaries and the uppermost reaches of the main stem (Fig. 2). Only three cases of infections were detected from non-spring-fed tributaries (Table 1). The optimal GLMM for the prevalence of leeches was the full model consisting of the all candidate variables (intercept coefficient = −4·17 ± 0·56, z-value = −7·48, P < 0·001), with a difference of AIC from the second one (ΔAIC = 4·9) consisted of only one variable (i.e. habitat type). Leech prevalence was more frequent in tributaries than in the main stem (partial coefficient of main stem site for spring-fed tributary = −2·13 ± 0·47, z-value = −4·53, P < 0·001; partial coefficient of non-spring-fed tributary for spring-fed one = −5·33 ± 0·62, z-value = −8·56, P < 0·001). The possibility of the parasite found in the sites also reflected a tendency to slightly increase with the geographic distance from the inlet (partial coefficient = 0·04 ± 0·017, z-value = 2·61, P = 0·009).
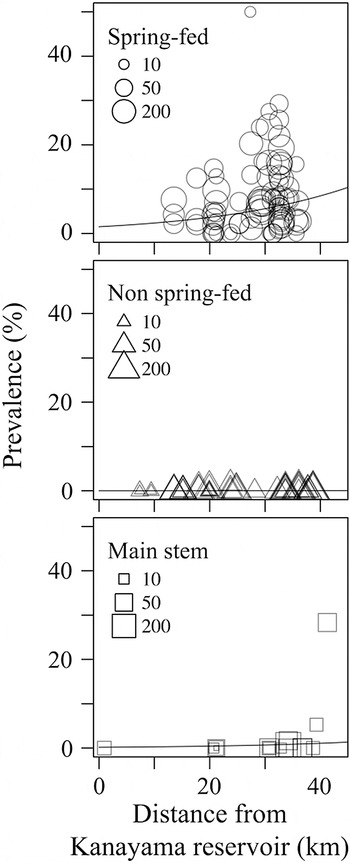
Fig. 2. Spatial distribution of the fish leech Taimenobdella amurensis in the river system. Each plot indicates the leech prevalence from one sampling site and term. Size of each shape represents the sample size of the all fish examined.
Additional small-scale surveys in the Shimizusawa Creek observed a clear decrease in prevalence from upstream to downstream (Fig. 3A); with a higher prevalence found in the upper reaches of the creek. On the contrary, prevalence in the small tributaries showed similar levels between the upper and lower reaches (Fig. 3B). Furthermore, leech infection was never found outside of the tributaries, compared with inside (Fig. 3C).

Fig. 3. Habitat use in and around the preferred patches. (A) Longitudinal distributions within the creek and (B) within the small tributaries, and (C) occurrence between inside and outside of the tributary. The leech prevalence in all fishes examined was provided as infected/examined in (A). Abbreviations in (B) and (C) are site names provided in Fig. 1.
Abundance in the preferred habitat
The leech infection ranged from one to five individuals per host fish (Fig. 4A), with a mean 0·110 and deviance 0·146 in the prevalent sites (i.e. spring-fed tributaries). As a result of the model fitting, the NB distribution was selected as the optimal error distribution explaining the abundance data, representing the minimum AIC value among NB, ZIP (ΔAIC = 38·7) and ZINB (ΔAIC = 2·0) distributions. The optimal GLMM model consisted of all candidates of the explanatory variables (i.e. host body size, season and year) (intercept coefficient = −5·65 ± 0·30, z-value = −19·04, P < 0·001); leech abundance positively (but only slightly) associated with the body size of the most preferred host, i.e. Dolly Varden (partial coefficient = 0·023 ± 0·0012, z-value = 18·94, P < 0·001) (Fig. 4B); infection levels in Dolly Varden were higher in spring than in autumn (partial coefficient of spring for autumn = 2·30 ± 0·21, z-value = 11·15, P < 0·001) and were low in 2014–2016 compared with 2013 (partial coefficient of 2014 for 2013 = −0·80 ± 0·094, z-value = −8·54, P < 0·001; that of 2015 for 2013 = −0·96 ± 0·095, z-value = −10·10, P < 0·001; that of 2016 for 2013 = −1·20 ± 0·11, z-value = −10·95, P < 0·001) (Fig. 4C).

Fig. 4. Infection status of the leech on the preferred host, Dolly Varden, in the prevalent habitats (i.e. spring-fed tributaries). (A) Histogram of the leech infection; estimated parameters and 95% bootstrap confidence intervals (in parentheses, 10 000 permutation) of mean (μ) and variance (σ) in negative binomial distribution are provided. (B) A relationship between the number of worms and the host's FL, (C) seasonal and annual changes in the leech prevalence, (D) seasonal changes in the body size of the leech (n = 79, 4, 248, 21 in Spring and Autumn of 2013 and those of 2014, respectively).
Seasonal changes in the leech body size
Small individuals were mainly found in spring (mean ± s.d. = 6·80 ± 2·62 mm), whereas larger leeches were found in autumn (mean ± s.d. = 18·52 ± 6·03 mm) (Fig. 4D). This is most remarkable in 2013 with non-overlapping size distribution, whereas the difference was little smaller in 2014. The optimal LM, consisting of one explanatory variable (i.e. season), supported an approximately 2·72 (=18·52/6·80 mm) times difference in the leech body size on average (intercept coefficient = 6·88 ± 0·33, t-value = 21·02, P < 0·001; partial coefficient of autumn for spring = 11·72 ± 0·62, t-value = 18·90, P < 0·001).
Synchrony of the leech prevalence
As a result of pairwise cross-correlation calculated from 11 prevalent sites (consisted of the Shimizusawa Creek and 10 spring-fed tributaries) where the leech infection was continuously investigated in the spring season for 4 years, moderate level of synchrony was detected, with average correlation coefficient 0·315 and a 95% bootstrap confidence interval 0·145–0·479.
DISCUSSION
We demonstrated highly aggregated distribution in an ectoparasite at a small spatial scale (<30 km, within a single watershed), suggesting that local populations can exist at the tributary level and that individuals found in the main stem are due to sporadic passive dispersal depending on host movement. In this case, if we survey only in the main stem, we would consider the leech occurrence being low (2·3% prevalence in the whole, see also Nagasawa et al. Reference Nagasawa, Kikuchi, Arakane and Yusa2009), but if we survey in small spring-fed tributaries, which may be normally overlooked, they are not very uncommon. This kind of highly heterogeneous distribution may be a characteristic of many parasites with infrequent occurrence and/or low intensity (Kennedy, Reference Kennedy2012). At the same time, however, local population sizes of the fish leech may still be small due to the small sizes of tributaries. We discuss potential population structure and persistence of the ectoparasite, considering the host and parasite life cycles and the factors affecting the narrow suitable habitat.
Life cycle and habitat requirement of the parasitic leech
Taimenobdella amurensis is most likely to have an annual life cycle because small individuals were only found in spring and large individuals in autumn. Decrease in the prevalence from spring to autumn should include both mortality and detachment from the host after ripening and readying to spawn, probably on stream substrates (see Sawyer, Reference Sawyer1986). Temporal variations in growth are also suggested from the annual difference in size distributions (Fig. 4D). Timing and duration leeches can attach to the host would significantly affect growth rates, which depend on host density and environmental conditions (Sawyer, Reference Sawyer1986).
Although life history of freshwater fish leeches is poorly understood, one of the few good examples of salmon leeches, Cystobranchus salmositicus, shows a remarkable adaptation to host life history (Becker and Katz, Reference Becker and Katz1965). This leech has an annual life cycle adjusted to anadromous salmonids, with hatching from cocoons when host fishes return from sea to rivers in September for spawning, and it subsequently grows up and reproduces prior to the host's death in December (Becker and Katz, Reference Becker and Katz1965). Dolly Varden in this river undergo a similar life cycle as anadromous salmonids: age-1+ juveniles, biasing towards females, migrate from natal tributaries to the main stem to grow, and returned to tributaries for spawning after 0·5–2·5 years of feeding in the main stem (Koizumi et al. Reference Koizumi, Yamamoto and Maekawa2006a ; Ayer et al. Reference Ayer, Katahira, Fukui and Koizumi2017). In addition, the main stem is apparently unsuitable habitat for the leech, at least for reproduction, which acts similarly to the ocean for the salmon leech. In spring, age-0+ Dolly Varden are most abundant in spring-fed tributaries with some age-1+ fish (60–110 mm), whereas large migratory individuals (130–200 mm) move to the tributaries in autumn (Koizumi et al. Reference Koizumi, Yamamoto and Maekawa2006a ). Interestingly, leeches emerged in spring and were small enough to attach age-0+ fish, and large leeches were occasionally found on migratory individuals (unpublished data). Therefore, these leeches might partly adjust to the life history of Dolly Varden.
High aggregation of T. amurensis in small spring-fed tributaries and the uppermost reaches of the main stem suggest that some environmental factors limit the distribution of this leech. In fact, cold water, high dissolved oxygen and stable water current are required for some freshwater leeches (see Sawyer, Reference Sawyer1986), which are all traits related to spring-fed streams. In particular, because T. amurensis is a northern species (Epshtein, Reference Epshtein1964; Lukin, Reference Lukin1976; Furness et al. Reference Furness, Williams, Nagasawa and Burreson2007), one would expect the maximum temperature to be a limiting factor. However, non-spring-fed tributaries and the main stem in the present river system are generally <15 °C and T. amurensis can tolerate >20 °C for more than a week (H. Katahira, unpublished data). There might be a critical stage for the development of individuals that is related to water temperature (Amat-Valero et al. Reference Amat-Valero, Calero-Torralbo and Valera2013). For example, eggs might be unable to hatch under near-freezing temperatures, which could explain the concentration in spring-fed tributaries, where the winter stable temperature is relatively high.
The low occurrence of the leech in the main stem might also be due to the anti-parasite behaviours of fishes. In other fish–ectoparasite systems, avoidance responses to parasite presence by move to different microhabitats, such as near the surface and open areas, have been reported (Poulin and Fitzgerald, Reference Poulin and Fitzgerald1989; Poulin et al. Reference Poulin, Rau and Curtis1991; see also Curtis, Reference Curtis2014). In addition to this, scraping against the substrate because of irritation from ectoparasite infection is well described in cage culture (Shimura, Reference Shimura1983; Woo and Shariff, Reference Woo and Shariff1990). We have also observed scraping behaviour of Dolly Varden in an aquarium when leeches were attached (unpublished data). Thus, physical conditions in the main stem with a rocky bottom substrate and high-flow open areas, which is totally differed from that in the tributaries, may work to negatively impact leech existence.
High frequency of infection on Dolly Varden is apparently due to the parasite's habitat requirements. Spring-fed tributaries are mostly dominated by Dolly Varden (Koizumi and Maekawa, Reference Koizumi and Maekawa2004) possibly due to temperature-dependent competition (Taniguchi and Nakano, Reference Taniguchi and Nakano2000). When other fishes occurred in spring-fed tributaries or the cold upper main stem, the leech attached to other fishes, such as sculpin and white-spotted charr. It is also reported in another stream that T. amurensis attached to different host species, such as sticklebacks and freshwater gobies (Nagasawa et al. Reference Nagasawa, Kikuchi, Arakane and Yusa2009). Therefore, the aggregated distribution is not due to host specificity but habitat requirements.
Possible metapopulation structure in T. amurensis
Self-sustaining populations of T. amurensis may be expected in some large tributaries (e.g. the Shimizusawa Creek), but not in others. Because many tributaries are small, the population size of Dolly Varden, the main host fish, are also small with 30–100 adults or <1000 individuals including juveniles (Koizumi, Reference Koizumi2011; Koizumi and Shimatani, Reference Koizumi and Shimatani2016). Simply calculated from the mean abundance data of the parasite, there should be, at most, a few hundred individuals attached to host fishes in each tributary; the total population size would not be large, given the limited host resources. Piscicolid leeches are also known to deposit multiple clutches (i.e. cocoons containing eggs) on substrates (see Sawyer Reference Sawyer1986), but reproductive rate in the present leech may not be high, considering from restricted recruitment in the spring season. Thus, it is unlikely that the leeches could form self-sustaining populations in most of the small tributaries.
For small populations, immigrants from other patches have an important role in population persistence (Hartvigsen and Halvorsen, Reference Hartvigsen and Halvorsen1993, Reference Hartvigsen and Halvorsen1994; see also Kennedy, Reference Kennedy2001, Reference Kennedy2012). In this context, dispersal of Dolly Varden is a key transporter of the parasite. Genetic analysis indicated that gene flow occurs among neighbouring tributaries and dispersal distance of Dolly Varden may be 3–5 km (Koizumi et al. Reference Koizumi, Yamamoto and Maekawa2006b ). If the leech immigrants are supplied via the host dispersal, stable tributary populations of the leech could conceivably to be sustained for a long time. Although the data are not yet sufficient (i.e. 4 years in 11 sites) and a slightly decreasing trend was observed during the study period, a moderate level of synchrony was observed in leech population dynamics. This suggests that dispersal is not so high as to connect all tributary populations functioning as a single panmictic population, but not so low for the populations to be completely isolated from each other.
Host dispersion as a vehicle has a powerful role for parasite migration and exchanges among local individuals (Poulin, Reference Poulin2007a ). It thus seems obvious if a host species forms a metapopulation structure, its parasite also forms a metapopulation structure. Rather, the putative metapopulation structure of the present parasite may be more prominent than host fish with the possibility of frequent local extinction and re-colonization, because local population sizes are smaller and its vagility should be less than that of the host. Empirical evidence of host–parasite co-structuring is critically lacking and further research deserves attention.
Implications to ecological laws
As shown by one of the few known ecological laws in parasitism (Poulin, Reference Poulin2007b ), spatial aggregation in almost all macroparasites, including rarely found species with small mean abundance, is explained by a probability of a NB distribution (Shaw and Dobson, Reference Shaw and Dobson1995; Poulin, Reference Poulin2013). According to this law, zero-inflated data can be excluded, even in rarely found parasites, when field samplings are properly designed. The present leech infection follows a negative binominal distribution within a preferred habitat (i.e. spring-fed tributary), suggesting that our sampling detected the infectious hot spots of the leech while avoiding false zero data as much as possible. The few occurrences in the density survey in the Chitose River from 2001 to 2008 (Nagasawa et al. Reference Nagasawa, Kikuchi, Arakane and Yusa2009) appear to be due to the middle-main stem location of the aquarium apart from the main hot spot of the parasite. Since the Chitose River system is composed of spring-fed tributaries (Kawai et al. Reference Kawai, Ishiyama, Hasegawa and Nakamura2013), the patchy feature is also expected as similar to the present river system with an annually reproducing population of tributary.
In some common species, occurrences have been thought to continuously decrease along with river line from downstream to upstream (see Blasco-costa et al. Reference Blasco-costa, Koehler, Martin and Poulin2013), following the River Continuum Concept that explains the longitudinal gradient of biomass in various aquatic organisms (Vannote et al. Reference Vannote, Minshall, Cummins, Sedell and Cushing1980). The inverse trend in our case is clearly due to the specific habits of the leech preferring spring-fed conditions that are also found in the uppermost reaches where the river size is decreasing at branch level, such as the Shimizusawa Creek. The groundwater-rich environment in the headwater area is necessary to account for the ectoparasite infection as a probable factor. Further, the river ecosystem is characterized by not only the unidirectional factor from top to bottom, but also by discontinuous environmental factors (Poole, Reference Poole2002), such as riffle and pool structures originating from large woody debris (Fausch and Northcote, Reference Fausch and Northcote1992; Wonzell and Swanson, Reference Wonzell and Swanson1999), tributary confluences (Benda et al. Reference Benda, Poff, Miller, Dunne, Reeves, Pess and Pollock2004; Fernandes et al. Reference Fernandes, Podos and Lundberg2004) and stream connectivity related to patch adjacency (Lowe et al. Reference Lowe, Likens and Power2006). Since ectoparasite habits are sensitive to abiotic factors in addition to interactions with their hosts (Chubb, Reference Chubb1977; Sawyer, Reference Sawyer1986; Marcogliese and Cone, Reference Marcogliese and Cone1996; Kearn, Reference Kearn2004), hot spots of some species may be explained by such discontinuous fluvial environments within a drainage system, as well as under spring-fed conditions.
The patchy distribution of T. amurensis also indicates that the parasite burden differs between the habitat types. This heterogeneous spatial use deserves further attention, specifically in regard to local adaptations in the host–parasite system (Kalts and Shykoff, Reference Kalts and Shykoff1998). There is a possibility that specific selective pressure by the leech infection affects the host fish traits, especially in the spring-fed tributaries. For example, diversity of major histocompatibility complex in the local host populations can be changed with parasite presence (Wegner et al. Reference Wegner, Kalbe, Kurtz, Reusch and Milinski2003; Rauch et al. Reference Rauch, Kalbe and Reusch2006). Given the immunogenetic differences partly relating to the host demography (e.g. fitness; Kalbe et al. Reference Kalbe, Eizaguirre, Dankert, Reusch, Sommerfeld, Wegner and Milinski2009), life history traits in the preferred host Dolly Varden should be compared between the leech-abundant hot spots (i.e. spring-fed tributaries), fewer leech hot spots (i.e. the uppermost area of the main stem) and no leech environments (i.e. non spring-fed tributaries).
Conclusion
This large-scale survey demonstrated the heterogeneous spatial use of the rare stream parasite, T. amurensis, within a mountain watershed. This hot spot-dependent distribution further suggests that the parasite has a metapopulation structure at a small spatial scale, retained by immigrants transported via host migratory behaviour between the tributaries. Metapopulation structure is often presumed in many parasites due to the nature of patchy habitats (i.e. host individuals or specific areas) (Poulin, Reference Poulin2007a ; Criscione, Reference Criscione2008; McCoy, Reference McCoy2009), but empirical data remain limited, except for some common species (Bruyndonckx et al. Reference Bruyndonckx, Henry, Christe and Kerth2009; Dharmarajan et al. Reference Dharmarajan, Beasley, Beatty, Olson, Fike and Rhodes2016) or at large spatial scales (on the order of 100–1000 km) (McCoy et al. Reference McCoy, Boulinier, Tirard and Michalakis2003). The heterogeneous occurrence of parasites also provides a good opportunity to evaluate the local adaptation of host species (see Kalts and Shykoff, Reference Kalts and Shykoff1998). Due to the ease of collecting a large amount of data without sacrificing host species, the leech–fish system in this river may be an ideal strategy to study the ecology and evolution of host–parasite interactions.
ACKNOWLEDGEMENTS
We thank H. Omiya, an official staff of the town hall of Minami-Furano, for helping the field samplings. We also express our gratitude for the members of the laboratory of Animal Ecology, Graduate School of Environmental Earth Science, Hokkaido University, for assistance and providing useful comments to our investigation. We thank the editor and two anonymous reviewers for their kind and helpful comments.
FINANCIAL SUPPORT
This study was partially financially supported by Grant-in-Aid for JSPS Fellows to H.K. (grant number 254625) and by a Grant-in-Aid for Young Scientists (B) to I.K. (grant number 23770013).







