Introduction
The piroplasms are an important cause of disease in humans, domestic animals and some wildlife, although most piroplasms in wildlife demonstrate low pathogenicity for their natural host (Hunfeld et al. Reference Hunfeld, Hildebrant and Gray2008; Yabsley and Shock, Reference Yabsley and Shock2012). Most piroplasms with known life cycles use ixodid ticks as vectors (Hunfeld et al. Reference Hunfeld, Hildebrant and Gray2008) although vertical transmission has been noted as a possible alternative transmission route for some piroplasms (e.g., Babesia microti in laboratory mice and humans, Babesia gibsoni and Babesia canis canis in dogs and Babesia bovis in cows) (Yeruham et al. Reference Yeruham, Avidar, Aroch and Hadani2003; Fukumoto et al. Reference Fukumoto, Suzuki, Igarashi and Xuan2005; Joseph et al. Reference Joseph, Purtill, Wong, Munoz, Teal, Madison-Antenucci, Horowitz, Aguero-Rosenfeld, Moore, Abramowsky and Wormser2012; Mierzejewska et al. Reference Mierzejewska, Welc-Faleciak, Bednarska, Rodo and Bajer2014; Bednarska et al. Reference Bednarska, Bajer, Drozdowska, Mierzejewska, Tolkacz and Welc-Faleciak2015; Adaszek et al. Reference Adaszek, Obara-Galek, Piech, Winiarchzyk, Kalinowski and Winiarczyk2016; Costa et al. Reference Costa, de Magalhães, de Oliveira, Carvalho, de Almeida, Machado and Munhoz2016). In addition, fighting and intermixing of individuals’ blood has been associated with direct transmission of B. gibsoni between fighting dogs (Yeagley et al. Reference Yeagley, Reichard, Hempstead, Allen, Parsons, White, Little and Meinkoth2009).
Babesia infections in raccoons have been reported sporadically throughout the Eastern and Midwestern USA (Schaffer et al. Reference Schaffer, Hanson, Davidson and Nettles1978; Anderson et al. Reference Anderson, Magnarelli and Sulze1981; Telford and Forrester, Reference Telford and Forrester1991; Birkenheuer et al. Reference Birkenheuer, Whittington, Neel, Large, Barger, Levy and Breitschwerdt2006, Reference Birkenheuer, Marr, Hladio and Acton2007; Clark et al. Reference Clark, Savick and Butler2012). However, there are currently little data on the pathogenicity of piroplasm infections in raccoons. A survey of raccoons from Japan with splenomegaly, a pathologic consequence of Babesia infections in other species, found that 8% (2/24) were positive for Babesia spp.; however, only raccoons with splenomegaly were tested (Kawabuchi et al. Reference Kawabuchi, Tsuji, Sado, Matoba, Asakawa and Ishihara2005; Adaszek et al. Reference Adaszek, Obara-Galek, Piech, Winiarchzyk, Kalinowski and Winiarczyk2016). Reports of Babesia infections in raccoons are generally based on older studies that utilized blood smears for piroplasms detection (Schaffer et al. Reference Schaffer, Hanson, Davidson and Nettles1978; Anderson et al. Reference Anderson, Magnarelli and Sulze1981; Telford and Forrester, Reference Telford and Forrester1991).
Two species of morphologically similar, but molecularly distinct, piroplasms have been reported from Florida, Massachusetts, North Carolina and Illinois (Goethert and Telford, Reference Goethert and Telford2003; Birkenheuer et al. Reference Birkenheuer, Whittington, Neel, Large, Barger, Levy and Breitschwerdt2006; Birkenheuer et al. Reference Birkenheuer, Marr, Hladio and Acton2007; Clark et al. Reference Clark, Savick and Butler2012). One is a species related to B. microti (hereafter called B. microti-like species) and the other is a Babesia sensu stricto species now referred to as B. lotori (also called Babesia sp. AJB-2006) (Anderson et al. Reference Anderson, Magnarelli and Sulze1981; Birkenheuer et al. Reference Birkenheuer, Marr, Hladio and Acton2007). The phylogenetic relationships of the piroplasms are under debate, but the B. microti clade is considered by many researchers to be a novel genus and likely has many unique biological characteristics (Lack et al. Reference Lack, Reichard and Van Den Bussche2012; Schreeg et al. Reference Schreeg, Marr, Tarigo, Cohn, Bird, Scholl, Levy, Weigmann and Birkenheuer2016). Without molecular characterization, it is unknown which or both of these species are present in infected raccoons. Furthermore, the prevalence and distribution of these two raccoon piroplasms is poorly known. Outside of the USA, via molecular assays, a low prevalence of B. microti-like parasites and at least one Babesia sensu stricto species have been reported from raccoons introduced to Japan (Kawabuchi et al. Reference Kawabuchi, Tsuji, Sado, Matoba, Asakawa and Ishihara2005; Jinnai et al. Reference Jinnai, Kawabuchi-Kurata, Tsuji, Nakajima, Fujisawa, Nagata, Koide, Matoba, Asakawa, Takahashi and Ishihara2009).
Currently, the transmission route is unknown for both Babesia spp. of raccoons but it is presumed to be via ixodid ticks as most piroplasms are transmitted by ixodid ticks (Uilenberg, Reference Uilenberg2006; Hunfeld et al. Reference Hunfeld, Hildebrant and Gray2008). Raccoons are commonly infested with several tick species including Ixodes texanus, Ixodes cookei, Ixodes scapularis, Ixodes affinis, Dermacentor variabilis, Amblyomma americanum and A. maculatum, but the geographic distribution and seasonality of many of these tick species varies (Dennis et al. Reference Dennis, Durden and Snyder1994; Ouellette et al. Reference Ouellette, Apperson, Howard, Evans and Levine1997; Yabsley et al. Reference Yabsley, Murphy, Luttrell, Little, Massung, Stallknecht, Conti, Blackmore and Durden2008). In North Carolina, both piroplasms are found in raccoons in high prevalences (Birkenheuer et al. Reference Birkenheuer, Whittington, Neel, Large, Barger, Levy and Breitschwerdt2006), so the vector, if there is one, is presumed to be a common tick species found on raccoons. Unfortunately, all of the tick species noted above are found in North Carolina so testing of raccoons from various parts of North America would be needed to better identify possible tick vectors (Ouellette et al. Reference Ouellette, Apperson, Howard, Evans and Levine1997; Birkenheuer et al. Reference Birkenheuer, Whittington, Neel, Large, Barger, Levy and Breitschwerdt2006). However, there is the possibility of alternative transmission routes.
Numerous questions remain regarding the natural history of piroplasm infections in raccoons, including the prevalence and diversity of different piroplasm species infecting raccoons, modes of transmission and pathogenicity. Thus, our objectives were to determine the prevalence of Babesia in young raccoons under the age of 6 weeks old and, if they occurred, what species of Babesia were present. We hypothesized that young raccoons would be infected with at least the two species of piroplasms previously reported in raccoons as the prevalence of both are very high in adult raccoons. In addition, we calculated a spleen:body weight ratio to determine if raccoons infected with Babesia spp. had larger spleens because splenomegaly has been associated with piroplasm infections. Finally, we examined the raccoons to determine if an infestation of ticks occurred while still in the nest, as raccoons younger than 6 weeks old typically do not venture from the nest (Schneider et al. Reference Schneider, Mech and Tester1971; Gehrt and Fritzell, Reference Gehrt and Fritzell1998). The current vector(s) of any piroplasms of raccoons are unknown but if piroplasm infections were noted in young raccoons, I. texanus, a tick species that is transmitted among raccoons in the nest (Anderson et al. Reference Anderson, Magnarelli and Sulze1981), would be expected to be present and would possibly be associated with transmission. However, it is also possible that other tick species may be transmitted to young raccoons within the nest environment. We sampled young raccoons in Minnesota and Colorado where we have previously detected Babesia infections in adult raccoons (Garrett and Yabsley, unpublished data).
Methods
Sample collection
From April to November of 2016, samples were collected from fetal, neonatal or juvenile raccoons admitted to two rehabilitation facilities: the Wildlife Rehabilitation Center of Minnesota (Roseville, MN) and the Greenwood Wildlife Rehabilitation Center in Colorado (Longmont, CO). The raccoons sampled were presented to the centres deceased, died while in care, or were euthanized due to poor prognosis. Raccoons were frozen immediately after death to ensure no decomposition occurred and shipped to the Southeastern Cooperative Wildlife Disease Study (Athens, GA) where they were processed. Raccoons were examined for ectoparasites, which if found, were preserved in 70% ethanol until identification. Ticks were identified morphologically using published keys (Keirans and Litwak, Reference Keirans and Litwak1989; Durden and Keirans, Reference Durden and Keirans1996; Guglielmone et al. Reference Guglielmone, Robbins, Apanaskevich, Petney, Estrada-Peña and Horak2014) or by using molecular methods as described below. Spleens were removed after examination, weighed and re-frozen at −20 °C until testing.
Data collected from each raccoon included weight, body length, sex and estimated age based on tooth eruption (Montgomery, Reference Montgomery1964). For a limited number of raccoons, age was approximated based on weight because of missing or damaged teeth. The age of remaining raccoons was also estimated based on weight and both methods provided similar results (data not shown). Although no animals were euthanized for the purposes of this study, the collection of biological samples for pathogen testing was reviewed and approved by UGA's Institutional Animal Care and Use Committee (A2014 10–018).
Molecular testing
Genomic DNA was extracted from ~10 mg of spleen using a commercial kit per the manufacturer's instructions (DNEasy Blood and Tissue kit, Qiagen, Hilden, Germany). Two different PCR (polymerase chain reaction) assays targeting the V4 region of the 18S rRNA gene of Babesia were used as described (Birkenheuer et al. Reference Birkenheuer, Levy and Breitschwerdt2003, Reference Birkenheuer, Marr, Hladio and Acton2007). One set of primers, BMlikeF (5′-CTGCCTTATCATTAATTTCGCTTCCGAACG) and 793–772R (5′-ATGCCCCCAACCGTTCCTATTA), targets Babesia parasites in the B. microti-like clade. Molecular analyses were conducted on a BioRad DNA Engine Peltier Thermal Cycler (Bio-Rad Laboratories Incorporated, Foster City, CA). Cycling parameters were 94 °C for 5 min followed by 49 cycles of 94 °C for 45 s, 56 °C for 45 s and 72 °C for 45 s, with a final extension of 72 °C for 5 min. The other set of primers, 455–479F (5′-GTCTTGTAATTGG-AATGATGGTGAC) and 793–772R, were used to detect Babesia sensu stricto species. Cycling parameters were 94 °C for 3 min followed by 44 cycles of 94 °C for 30 s, 60 °C for 30 s, 72 °C for 30 s, with a final extension of 72 °C for 5 min.
Precautions were taken to prevent and detect contamination including the performance of DNA extraction, PCR reaction setup, and product analysis in distinct, designated areas. Negative water controls were included in each set of DNA extractions. For each batch of PCR reactions, the extraction negative control, a new water negative control and a positive control (DNA sample from a pooled blood sample with the sequenced-confirmed presence of B. lotori and B. microti-like sp.) were included. Amplicons were observed in a GelRed stained 1.5% agarose gel. Gels were run for an extended period of time to ensure the reliable distinction between amplicon sizes.
Because the screening PCR assays amplify a small amplicon not ideal for species identification, especially within the Babesia sensu stricto group, 11 samples positive with the Babesia sensu stricto group 18S screening primer set were also tested using a PCR targeting the cytochrome c oxidase subunit 1 (cox1) region and products were sequenced to identify species present. Primers Babcox1F (5′-GGAAGTGGWACWGG-WTGGAC) and Babcox1R (5′-TTCGGTATTGCATGCCTTG) were used and cycling parameters were 95 °C for 5 min followed by 45 cycles of 95 °C for 20 s, 50 °C for 30 s, 68 °C for 1 min and 30 s, and a final extension of 72 °C for 5 min (Schreeg et al. Reference Schreeg, Marr, Tarigo, Cohn, Bird, Scholl, Levy, Weigmann and Birkenheuer2016). For eight samples that were coinfected and a Babesia sensu stricto sequence was not obtained using the 18S screening or cox1 gene protocols, we conducted an additional PCR using Babesia sensu stricto-specific primers that target two regions of the large subunit rRNA (LSU) gene fragment (lsu5 and lsu4) (Qurollo et al. Reference Qurollo, Archer, Schreeg, Marr, Birkenheuer, Haney, Thomas and Breitschwerdt2017).
To confirm the presence of B. microti-like sp. in a subset of samples the BMlikeF/793–772R amplicon was sequenced (nine samples) or a larger region of the 18S rRNA gene was amplified with primers 522F and 1661R and sequenced (four samples) (Birkenheuer et al. Reference Birkenheuer, Marr, Hladio and Acton2007).
Amplicons were purified from an agarose gel using a gel-purification kit (Qiagen) and bi-directionally sequenced at the University of Georgia Genomics Facility (Athens, GA). Sequences were cleaned using the Geneious program (Biomatters Limited, Auckland New, Zealand) and consensus sequences compared with other Babesia sequences in GenBank.
Some Ixodes ticks were damaged during removal and could not be identified to species using morphologic characteristics. Tick DNA was extracted and amplified as described (Gleim et al. Reference Gleim, Conner, Berghaus, Levin, Zemtsova and Yabsley2014). Primers 16S − 1 (5′-CCGGTCTGAACTCAGATCAAGT) and 16S + 2 (5′-TTGGGCAAGAAGACCCTATGAA) targeting the 16S rRNA gene were used and cycling parameters were 94 °C for 2 min followed by 40 cycles of 94 °C for 30 s, 45 °C for 30 s, 72 °C for 1 min and a final extension of 72 °C for 5 min. Amplicons were sequenced as described above.
Statistical analyses
A Fisher's exact test was used to compare the prevalence of the B. microti-like sp. and Babesia sensu stricto. In order to determine the relationships between parasite prevalence and the different variables, a generalized linear model (GLM) was utilized and the data log transformed. The variables measured included sex, age (by week), spleen:body weight ratio and whether or not ticks were present. A separate GLM was performed for the B. microti-like sp. and Babesia sensu stricto datasets.
Results
A total of 106 young raccoons from Minnesota (n = 83) and Colorado (n = 23) were included in the study and 66% (70/106) were infected with Babesia. The prevalence of B. microti-like sp. [66/106 (62%)] was significantly higher than that of Babesia sensu stricto [11/106 (10%) (P = 0.0001)]. A total of eight (7.5%) raccoons had coinfections. For the B. microti-like sp., infections were detected in individuals as young as 1 week of age and prevalence was high in all age groups (Table 1). For the Babesia sensu stricto group, infections were first noted at 2 weeks of age but prevalence was low, while prevalence in raccoons that were 6 weeks or older was 40% (Table 1). Table 2 shows the data for the 59 raccoons from Minnesota that were admitted in identifiable litters. In general, the prevalence of Babesia within litters was high but for most litters, not all individuals were infected (Table 2).
Table 1. Prevalence of Babesia spp. in young raccoons from Colorado and Minnesota, by age classa
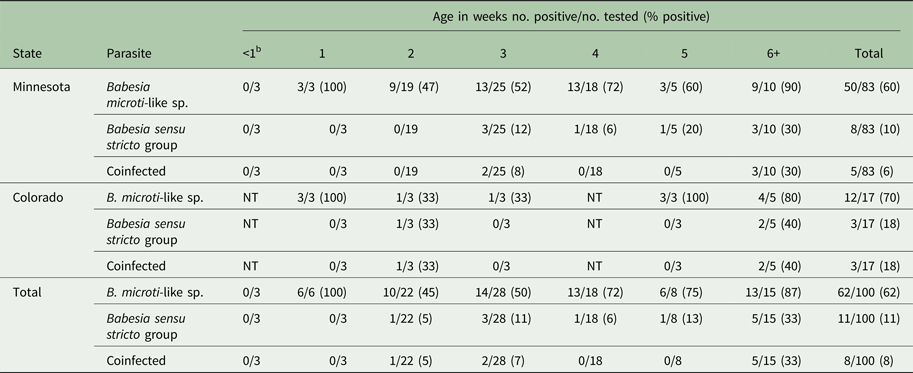
a Age was not available for six raccoons and were not included in the table.
b These three raccoons were near-term and removed via caesarian section from a deceased female.
Table 2. Data on Babesia infections and ticks on raccoons from identifiable litters from Minnesota.
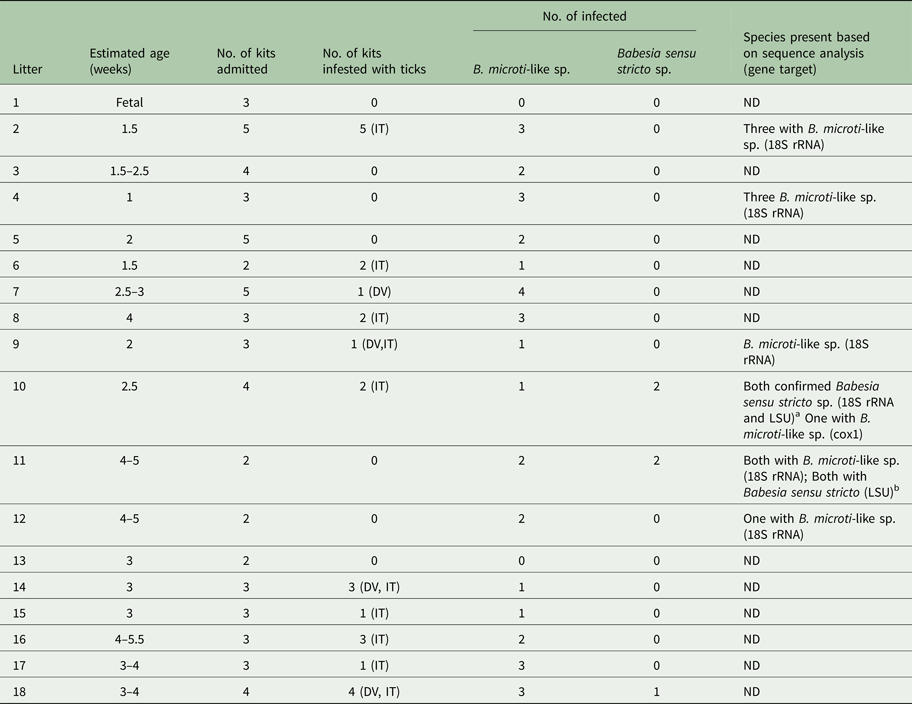
Table 3. Summary of PCR and sequence results for 11 raccoons positive for Babesia sensu stricto (eight coinfected with a B. microti-like sp. based on 18S rRNA screening PCR and three raccoons infected with only Babesia sensu stricto).
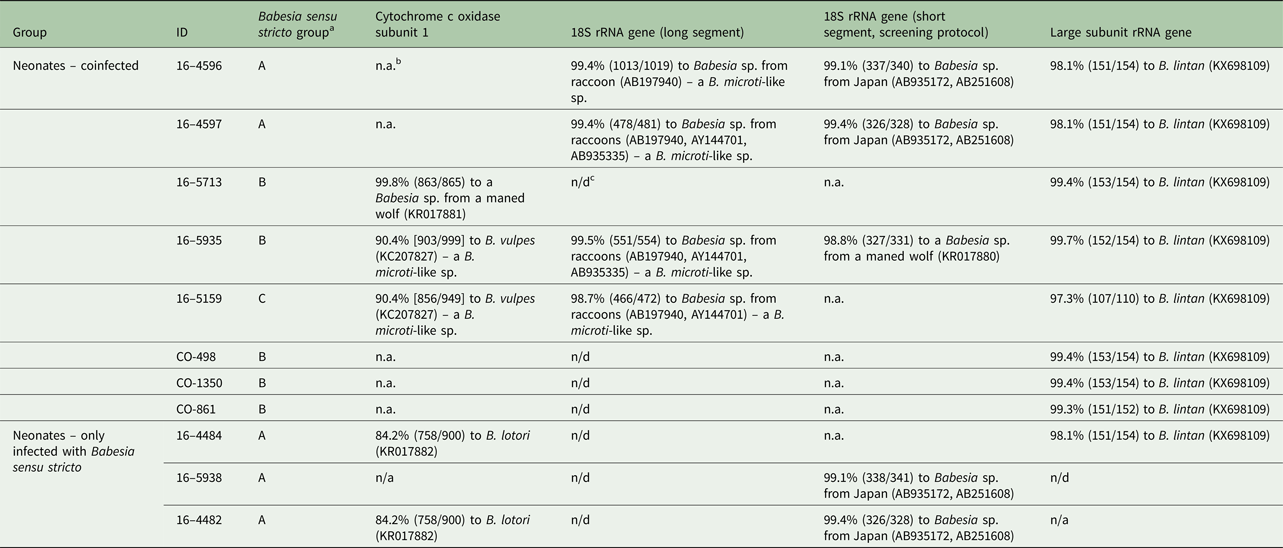
a Three groups of Babesia sensu stricto were identified based on sequences of the three gene targets.
b n.a., not available (PCR either was negative or we were unable to get a clean sequence).
c n/d, not done.
Eight of the 11 Babesia sensu stricto samples amplified with the cox1 PCR protocol but only five provided good quality sequences (all from Minnesota). One sequence was most similar (99.8%) to a Babesia sp. reported from a captive maned wolf (Chrysocyon brachyurus) (KR017881) but was also 98.3% similar to Babesia lotori (accessioned as Babesia sp. AJB-2006, KR017882) of raccoons. Two other sequences, from the same litter of raccoons, were identical to each other and, although they were most similar to B. lotori, they only shared 84.2% similarity (Table 2). The remaining two sequences were most similar (90.4%) to B. vulpes (a B. microti-like sp. of fox and dogs, accessed in GenBank as Babesia sp. MES-2012, KC207827); however, these sequences were identical to unpublished sequences of the B. microti-like sp. of raccoons (Garrett and Yabsley, unpublished data). Both of these raccoons were coinfected with a Babesia sensu stricto based on the screening 18S PCR; one (16–5935) of which had a partial 18S sequence to confirm Babesia sensu stricto infection (Table 3). All eight of the raccoons that were coinfected samples were successfully amplified using the LSU rRNA gene PCR assay. These LSU sequences were (97.3–99.7%) similar to Babesia lintan (Table 3). However, based on sequences from the cox1, 18S rRNA and LSU gene sequences, there were three distinct groups of Babesia sensu stricto (Table 3). Unique cox1 and 18S rRNA gene sequences were submitted to GenBank (accession numbers MG986879–MG986888).
Many of the samples positive for the B. microti-like sp. using the screen PCR did not produce amplicons with the cox1 PCR protocol, so to confirm infections with this species, we sequenced amplicons from the two different 18S PCR protocols. Near full-length 18S rRNA sequences were acquired for four raccoons and shorter 18S rRNA sequences were obtained for six additional raccoons, including the three raccoons in the 1-week-old litter (Table 2). All of these sequences were identical or most similar (>99.5%) to the B. microti-like sp. sequences from raccoons available in GenBank (AB197940, AB935335 and AY144701).
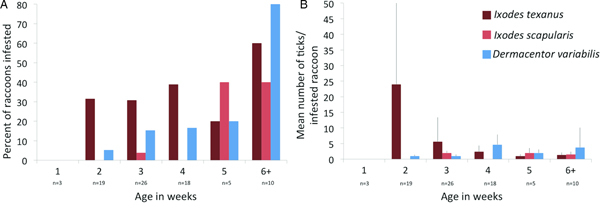
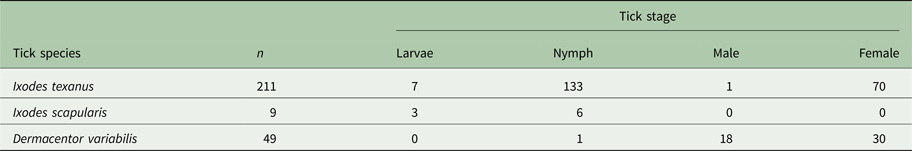
Three tick species were found on young raccoons including I. texanus, I. scapularis and D. variabilis, all from raccoons from Minnesota (Table 4). Damaged ticks that could not be identified to species based on morphological characteristics were identified as I. texanus using PCR and sequence analysis. All life stages of I. texanus were detected while only larvae and nymphs of I. scapularis were found. Infestation with I. texanus was first noted on raccoons at 2 weeks of age and the infestation prevalence was similar for all age groups (Fig. 1A). In contrast, I. scapularis infestations were primarily noted in raccoons older than 5 weeks of age, although a single 3-week-old raccoon was infested with two I. scapularis nymphs (Fig. 1A). Infestation prevalence for D. variabilis increased with age (Fig. 1A). The mean number of I. texanus collected from infested raccoons was highest for raccoons in the 2-week age group with two individuals having 54 and 64 ticks, respectively (Fig. 1B). Other than those two raccoons with high I. texanus infestations, tick burdens were generally low with a maximum number of ticks collected from an individual being three I. scapularis and 13 D. variabilis. Most ticks were found in the ears or on the face (Fig. 2) although two raccoons had ticks present on multiple parts of the body. Presence of ticks was not a significant predictor variable for infection with either piroplasm (B. microti-like sp.: P = 0.2393; Babesia sensu stricto: P = 0.3604).
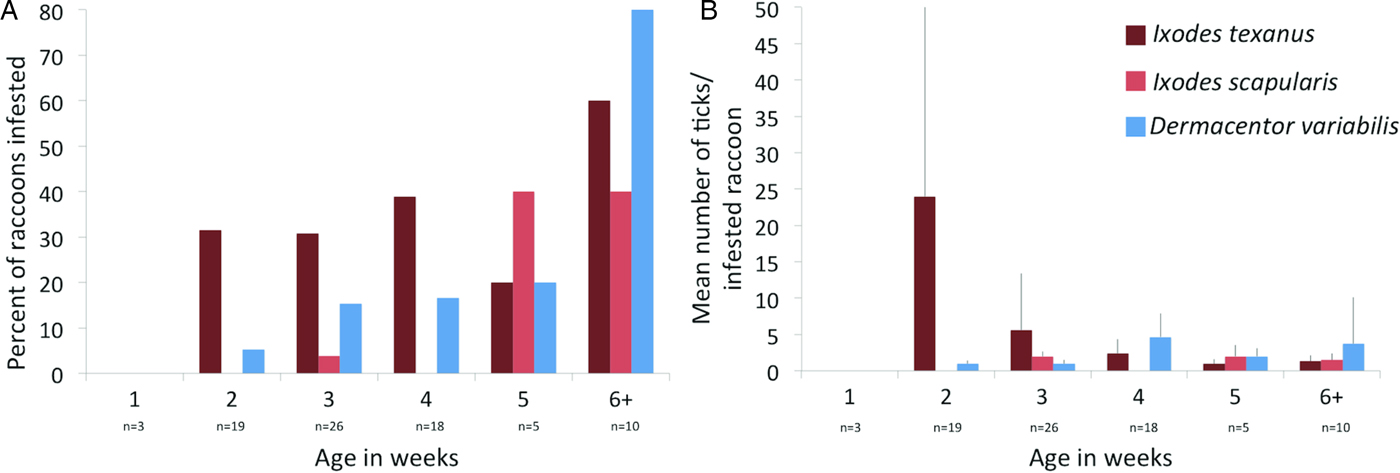
Fig. 1. (A) Per cent of young raccoon infested with ticks by age class in weeks (number of raccoons sampled in each age class show below age). (B) Average number of ticks from infested raccoons in each age class in weeks. Number of raccoons sampled is the same as in (A).
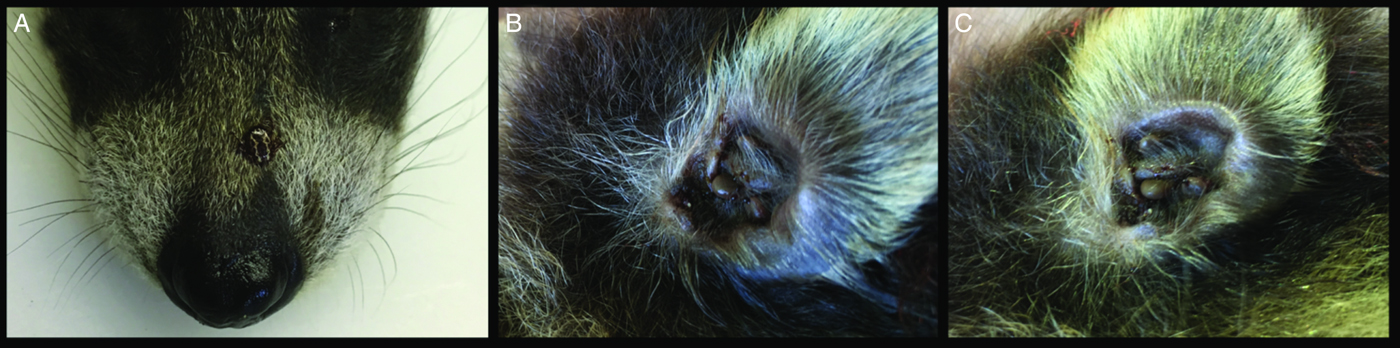
Fig. 2. Ticks on a 1.5-week-old raccoon. (A) An adult Dermacentor variabilis on snout. (B) Several nymphal and adult Ixodes texanus in an ear. (C) Several adult I. texanus in an ear.
Table 4. Number and stage of ticks collected from young raccoons from Minnesota
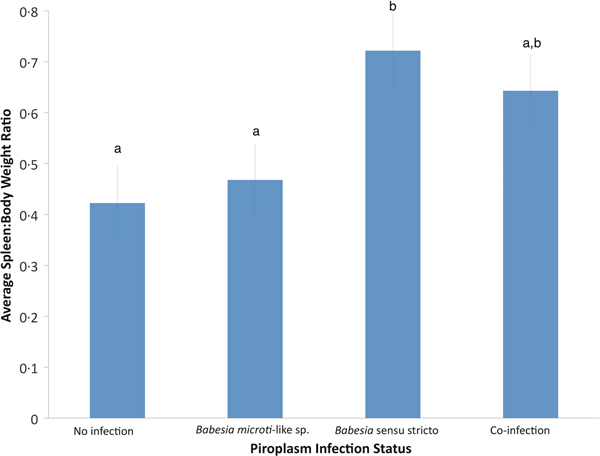
Using GLM, the only significant variable for raccoons infected with B. microti-like sp. was age (P = 0.0059). According to the GLM, infection of raccoons with Babesia sensu stricto was associated with age (P = 0.0017) and spleen:body weight ratio (P = 0.0005). Also, raccoons with Babesia sensu stricto infections had significantly higher spleen:body weight ratios compared with raccoons infected with B. microti-like sp. only or those with no Babesia infection (P = 0.0008 and P < 0.0001, respectively) (Fig. 3). Although not significantly different from either group, coinfected raccoons had increased average spleen:body ratio (P = 0.374) (Fig. 3).
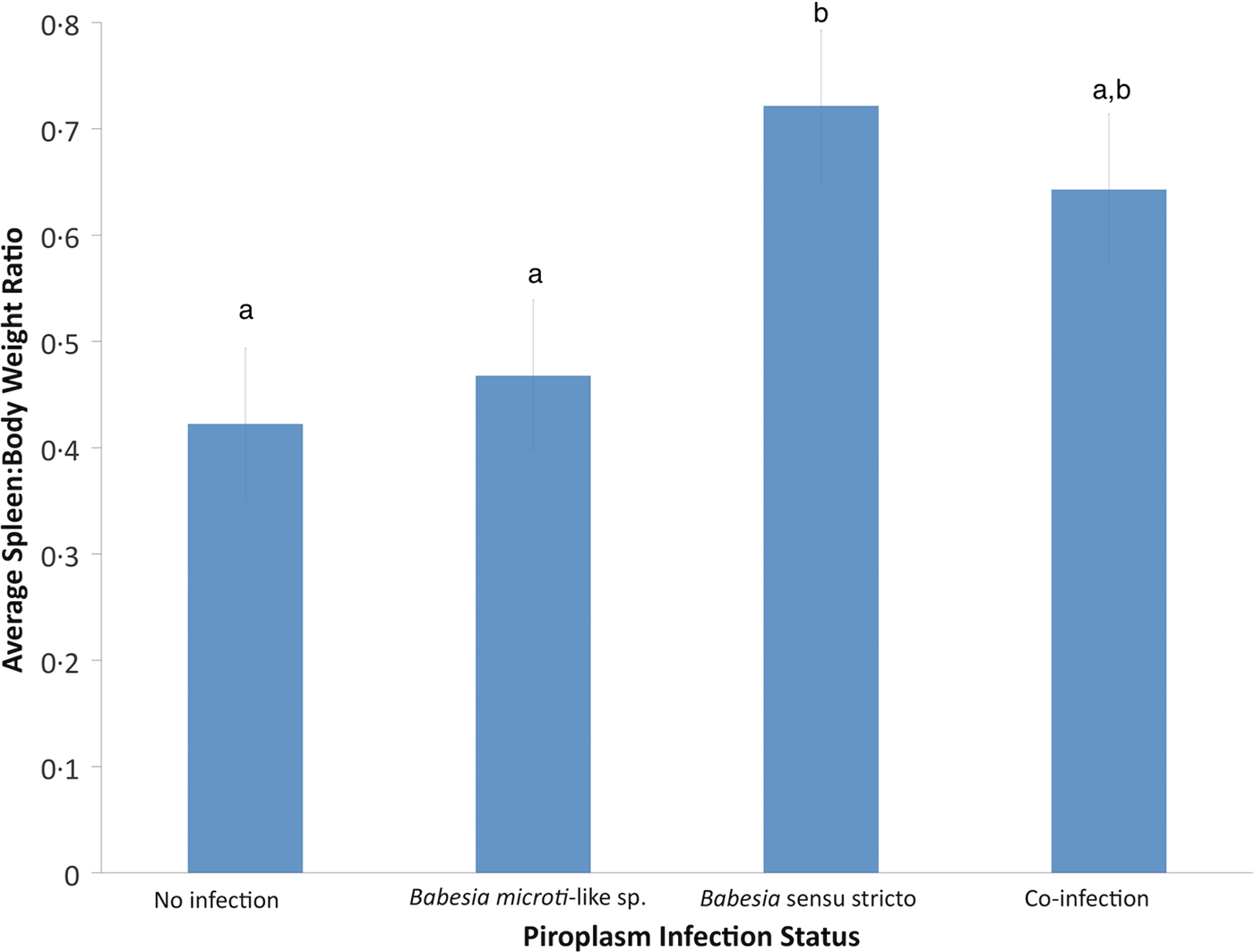
Fig. 3. Effects of Babesia infection on average spleen:body weight ratio of young raccoons with standard error bars. Different letters denote significant differences among groups.
Discussion
We detected Babesia infections in young raccoons from Minnesota and Colorado with the B. microti-like sp. detected in individuals as young as 1 week of age. There was a high prevalence of the B. microti-like sp. in raccoons from Minnesota and Colorado, and although Babesia sensu stricto infections were detected, the prevalence was much lower. We obtained sequence confirmation for the two piroplasms previously reported from raccoons (a B. microti-like sp. and B. lotori), but we also found possible novel Babesia spp. (groups B and C in Table 3). We also noted coinfections occurring in some young raccoons; however, the prevalence of coinfection in both states was much lower than previously reported for adult raccoons in North Carolina, but this is likely due to the lower prevalence of Babesia sensu stricto infections among the young raccoons we tested (Birkenheuer et al. Reference Birkenheuer, Whittington, Neel, Large, Barger, Levy and Breitschwerdt2006). These data extend the known range of B. lotori to Minnesota and confirms that Babesia sensu stricto spp. occur in Minnesota and the B. microti-like sp. occurs in Minnesota and Colorado.
The only previous study to investigate Babesia infections in young racccons was conducted in Connecticut and our data support those findings (Anderson et al. Reference Anderson, Magnarelli and Sulze1981). Anderson et al. (Reference Anderson, Magnarelli and Sulze1981) found that three of four young raccoons were positive for Babesia and nymphal I. texanus ticks were found on two of the raccoons. However, in the previous study, infections in raccoons were determined based on blood smear analysis and thus the species of Babesia present was unknown and the age of the raccoons was not specified. Generally, we found a high prevalence of Babesia among identifiable litters and although not all individuals in a litter were infected, these data were similar to data on vertical transmission of B. microti in voles (Tolkacz et al. Reference Tolkacz, Bednarska, Alsarraf, Dquznik, Grzybek, Welc-Faleciak, Behnke and Bajer2017).
A primary goal of our study was to determine if young raccoons were infected with Babesia and investigate the possible role of vertical transmission as a route of infection. However, because we also detected a high prevalence of tick infestation on raccoons from Minnesota, it is unknown if the infections in young raccoons were acquired vertically from infected female raccoons or were due to infestation with ticks at a very young age. While we did not find ticks on raccoons younger than 2 weeks of age, our sample size for 1-week-old raccoons was limited so it is possible that raccoons become infested with ticks earlier than we noted. We also did not note any infection in the three fetal raccoons; however, this was also a small sample size and samples from the dam were not available for analysis.
The prepatent period is generally unknown for many piroplasms and varies by transmission route and detection method, so reported data may not be valid for raccoon-infecting piroplasms. For vertical transmission of B. microti, voles in Europe had a 3-week prepatent period and experimentally infected BALB/c laboratory mice had a 20-day prepatent period (Bednarska et al. Reference Bednarska, Bajer, Drozdowska, Mierzejewska, Tolkacz and Welc-Faleciak2015; Tolkacz et al. Reference Tolkacz, Bednarska, Alsarraf, Dquznik, Grzybek, Welc-Faleciak, Behnke and Bajer2017). Our data suggest that some Babesia infections may be acquired due to vertical transmission, as we detected B. microti-like sp. infections in raccoons as young as 1 week old which is generally shorter than prepatent periods associated with tick transmission (e.g., 13–28 days for B. microti to rhesus macaques (Macaca mulatta) and 13–17 days to BALB/c mice) (Ruebush et al. Reference Ruebush, Piesman, Collins, Spielman and Warren1981; Li et al. Reference Li, Zhu, Zhang, Zhang and Zhou2016).
For Babesia sensu stricto spp., short prepatent periods after exposure of hosts to infected ticks have been documented. The prepatent period for B. canis transmitted to dogs by Rhipicephalus sanguineus was 6 days post-infection, whereas cattle became infected with Babesia major within 9–15 days after exposure to infected Haemaphysalis punctate (Paraense, Reference Paraense1949; Yin et al. Reference Yin, Lu, Luo, Zhang, Lu and Dou1996). Vertical transmission of Babesia sensu stricto sp. has also been noted with beagle puppies, whose mother was intravenously inoculated with B. gibsoni prior to mating, showing infection after 14 days (Fukumoto et al. Reference Fukumoto, Suzuki, Igarashi and Xuan2005). Other cases of vertical transmission of Babesia sensu stricto sp. have been reported in puppies infected with Babesia canis 6 weeks after birth and in puppies with clinical signs after 8 weeks from presumed vertical transmission of B. canis canis (Mierzejewska et al. Reference Mierzejewska, Welc-Faleciak, Bednarska, Rodo and Bajer2014; Adaszek et al. Reference Adaszek, Obara-Galek, Piech, Winiarchzyk, Kalinowski and Winiarczyk2016). Because infections of raccoons with Babesia sensu stricto were not noted until at least 2–3 weeks of age, which is within the time frame of tick-transmitted prepatent periods, it is possible that these two groups of Babesia, B. microti-like sp. and Babesia sensu stricto sp., utilize different transmission strategies.
Ticks, specifically ixodid ticks, are the presumed vectors for Babesia sp. and are important to discuss when considering the lifecycle of these raccoon piroplasms (Hunfeld et al. Reference Hunfeld, Hildebrant and Gray2008). The tick species found on our young raccoons from Minnesota are commonly reported on raccoons (Dennis et al. Reference Dennis, Durden and Snyder1994; Ouellette et al. Reference Ouellette, Apperson, Howard, Evans and Levine1997; Hersh et al. Reference Hersh, Tibbetts, Strauss, Ostfeld and Keesing2012). Ixodes texanus was the most common and abundant tick found on the raccoons which was expected as this species is found on host's year-around and is assumed to be acquired within the nests of their vertebrate hosts (Sonenshine, Reference Sonenshine1993; Dharmarajan et al. Reference Dharmarajan, Beasley, Beatty, Olson, Fike and Rhodes2016). This tick species has a widespread distribution in the USA (Eastern and Midwestern USA, California, and Alaska and likely many states in between) (Ouellette et al. Reference Ouellette, Apperson, Howard, Evans and Levine1997; Gabriel et al. Reference Gabriel, Brown, Foley, Higley and Botzler2009; Durden et al. Reference Durden, Beckmen and Gerlach2016). The other two species found on our raccoons included D. variablis (restricted to the Eastern USA and in isolated populations in California) and I. scapularis (restricted to the Eastern USA) (Bishopp and Trembley, Reference Bishopp and Trembley1945). Larval and nymphal stages of I. scapularis feed on a wide range of small to medium-sized hosts (mammals, birds, lizards), including raccoons, and this species is an important vector of Borrelia burgdorferi, the causative agent of Lyme disease, and B. microti, the primary causative agent of human babesiosis in the USA (Hersh et al. Reference Hersh, Tibbetts, Strauss, Ostfeld and Keesing2012). In our study, only larvae and nymphs of I. scapularis were found on raccoons and in very low numbers, most likely because of the earlier seasonal activity of these stages compared with adults, which are more often found on hosts in fall and winter (Bishopp and Trembley, Reference Bishopp and Trembley1945). Raccoons younger than 6–8 weeks most likely acquire ticks from the mother, as young of this age do not typically venture from the nest, while older individuals (7–10-week-old young) are more active and can become infested with ticks outside of the nest (Schneider et al. Reference Schneider, Mech and Tester1971; Gehrt and Fritzell, Reference Gehrt and Fritzell1998).
Piroplasm infections in most species of wildlife are considered to be of low pathogenicity, although under certain circumstances (e.g., coinfections, immunosuppression, stress, climate factors, etc.) they may cause disease (Penzhorn, Reference Penzhorn2006; Yabsley and Shock, Reference Yabsley and Shock2012). Examples include babesiosis in African lions suffering from a concurrent canine distemper virus outbreak and decreased food availability, and the development of fatal babesiosis in black rhinoceros due to the stress of capture for translocation efforts (Penzhorn, Reference Penzhorn2006; Munson et al. Reference Munson, Terio, Kock, Mlengeya, Roelke, Dubovi, Summers, Sinclair and Packer2008). In raccoons, Babesia infections are presumed to be of little clinical significance but most reports are surveys of healthy free-ranging adults. One possible clinical case in a raccoon was a single juvenile raccoon from Illinois that was infected with B. lotori (Birkenheuer et al. Reference Birkenheuer, Whittington, Neel, Large, Barger, Levy and Breitschwerdt2006). The raccoon was found non-ambulatory with pronounced anaemia, hypoproteinaemia, hypalbuminaemia and elevated alanine aminotransferase with rare intraerythrocytic Babesia parasites. It was treated for Babesia and released; however, it is unknown if it was the Babesia infection that caused the clinical signs or if they were the results of a secondary infection (Birkenheuer et al. Reference Birkenheuer, Whittington, Neel, Large, Barger, Levy and Breitschwerdt2006). In general, clinical disease is likely to be more pronounced in young animals. Studies on vertical transmission of piroplasms in several hosts indicate that clinical signs in infected young generally occur between 17 and 25 days (Fukumoto et al. Reference Fukumoto, Suzuki, Igarashi and Xuan2005; Bednarska et al. Reference Bednarska, Bajer, Drozdowska, Mierzejewska, Tolkacz and Welc-Faleciak2015; Brown et al. Reference Brown, Shiel and Irwin2015; Adaszek et al. Reference Adaszek, Obara-Galek, Piech, Winiarchzyk, Kalinowski and Winiarczyk2016). Because our sampled animals were not available for antemortem testing, we used the ratio of spleen weight:body weight as a measure of possible disease. The association with splenomegaly and Babesia sensu stricto infections, but not with B. microti-like sp. infections, suggests that early infections with B. lotori or the possible novel Babesia sp. may cause clinical disease in young raccoons. Unfortunately, because these raccoons were dead on arrival or euthanized on entry, no clinical pathology data were collected nor was any histologic analysis done to determine the cause of death or illness (although many were admitted because they were orphaned, not because they were sick). Splenomegaly is one of many common findings of clinical babesiosis in many host species (Kawabuchi et al. Reference Kawabuchi, Tsuji, Sado, Matoba, Asakawa and Ishihara2005; Mierzejewska et al. Reference Mierzejewska, Welc-Faleciak, Bednarska, Rodo and Bajer2014; Solano-Gallego et al. Reference Solano-Gallego, Sainz, Roura, Estrada-Peña and Miró2016), including puppies that acquired B. canis infection through vertical transmission (Mierzejewska et al. Reference Mierzejewska, Welc-Faleciak, Bednarska, Rodo and Bajer2014). Babesia has been detected in raccoons with splenomegaly in Japan; however, only raccoons with splenomegaly were tested and the prevalence of Babesia was low, possibly because raccoons were introduced to Japan (Kawabuchi et al. Reference Kawabuchi, Tsuji, Sado, Matoba, Asakawa and Ishihara2005; Jinnai et al. Reference Jinnai, Kawabuchi-Kurata, Tsuji, Nakajima, Fujisawa, Nagata, Koide, Matoba, Asakawa, Takahashi and Ishihara2009). It is possible that Babesia spp. of certain wildlife may be more pathogenic than currently recognized but only impact very young animals that are rarely studied.
Because we obtained B. microti sequences with the cox1 PCR, this protocol can amplify both Babesia sensu stricto species and B. microti-like sp. Unfortunately, due to financial constraints, cloning of these coinfected samples was not possible for this study. However, there were no polymorphic bases present in the cox1 sequences although the sequences that failed may have been due to mixed amplicons. We did not PCR test ticks collected from raccoons for piroplasms because ticks were all potentially blood-fed so any positives could have occurred from ingestion of infected blood from the raccoon, the previous infection from another raccoon, or vertical transmission of piroplasms within infected ticks. One of our objectives was to investigate the possibility of vertical transmission of piroplasms to raccoons but since we detected ticks at a young age and dams were not available for testing, we could not determine if transmission of piroplasms occurred vertically or via tick transmission.
In summary, we show that several species of Babesia infect very young raccoons. In addition, we showed that several species of ticks are parasitizing these young raccoons, so it is currently unknown if these infections are a result of vertical transmission or tick-transmission due to early infestation. Finally, we note that young raccoons infected with Babesia sensu stricto spp. have a higher spleen:body weight ratio suggesting possible clinical disease associated with infection. Additional studies are needed to better understand the natural history, diversity and impact of Babesia infections in raccoons.
Acknowledgements
We thank Chris Cleveland, Brianna Williams and Maddy Pfaff for assistance with laboratory procedures and statistical analyses. We also thank the staff at both wildlife rehabilitation centres for their assistance with the project.
Financial support
Financial assistance was provided by the Warnell School of Forestry and Natural Resources (support of K.B.G.) and the sponsorship of the Southeastern Cooperative Wildlife Disease Study by the fish and wildlife agencies of Alabama, Arkansas, Florida, Georgia, Kentucky, Kansas, Louisiana, Maryland, Mississippi, Missouri, Nebraska, North Carolina, Ohio, Oklahoma, Pennsylvania, South Carolina, Tennessee, Virginia and West Virginia, USA. Support from the states to SCWDS was provided in part by the Federal Aid to Wildlife Restoration Act (50 Stat. 917).
Conflict of interest
None.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional guides on the care and use of laboratory animals. Although no animals were euthanized for the purposes of this study, the collection of biological samples for pathogen testing was reviewed and approved by UGA's Institutional Animal Care and Use Committee (A2014 10-018).