INTRODUCTION
Reef fish contribute significantly to food security and income of coastal communities in many developing countries (Donner and Potere, Reference Donner and Potere2007; Hughes et al. Reference Hughes, Yau, Max, Petrovic, Davenport, Marshall and Cinner2012). Due to its high trade value and increasing demand on the international market, epinephelids belong to the most important fisheries resources, resulting in a continuously growing fishing pressure as well as aquaculture production in Indonesia (Yulianto et al. Reference Yulianto, Hammer, Wiryawan and Palm2015). Indonesia is the second largest grouper producer worldwide (FAO, 2015). Consequently, increasing attention is paid to studies concerning the ecology and biology of wild and cultivated epinephelids. In general, commercially important species are large, such as Epinephelus coioides and E. fuscoguttatus, with a maximum length up to 120 cm (Craig et al. Reference Craig, Sadovy de Mitcheson and Heemstra2011). Both species are relevant for fisheries as well as aquaculture, and have been intensively investigated for diseases and parasites in recent years (e.g. Rückert, Reference Rückert2006; Palm and Rückert, Reference Palm and Rückert2009; Kleinertz, Reference Kleinertz2010; Rückert et al. Reference Rückert, Klimpel and Palm2010; Palm et al. Reference Palm, Kleinertz and Rückert2011; Kleinertz et al. Reference Kleinertz, Damriyasa, Hagen, Theisen and Palm2014a ; Kleinertz and Palm, Reference Kleinertz and Palm2015; Neubert et al. Reference Neubert, Yulianto, Theisen, Kleinertz and Palm2016). In contrast, the knowledge on the parasite fauna of smaller epinephelids, like the white-streaked grouper Epinephelus ongus, is very limited.
E. ongus occurs in the Indo-West Pacific and inhabits coastal reefs as well as brackish water lagoons (Heemstra and Randall, Reference Heemstra and Randall1993). Ledges and caves in depths of five to 25 m are frequently used as shelter (Myers, Reference Myers1999). The diet consists of fish and crustaceans and a nocturnal feeding pattern can be assumed (Craig, Reference Craig2007). With a maximum length of about 40 cm (Craig et al. Reference Craig, Sadovy de Mitcheson and Heemstra2011) E. ongus is a relatively small member of the Epinephelidae (according to Smith and Craig (Reference Smith and Craig2007) the traditional taxon Serranidae is polyphyletic, resulting in the resurrection of the Epinephelidae). E. ongus increasingly contributes to the regular catches, and e.g. at the Naha fish market in Okinawa, Japan, it became the most landed epinephelid (Craig, Reference Craig2007). A similar development can be observed in Karimunjawa Islands, where overfishing of commercially important epinephelid species moves E. ongus more and more into the focus of fisheries. Thus, E. ongus became the most abundant landed epinephelid between 2009 and 2012, and contributed a high proportion to the total weight of landed epinephelids in the Karimunjawa archipelago (Fig. 1).
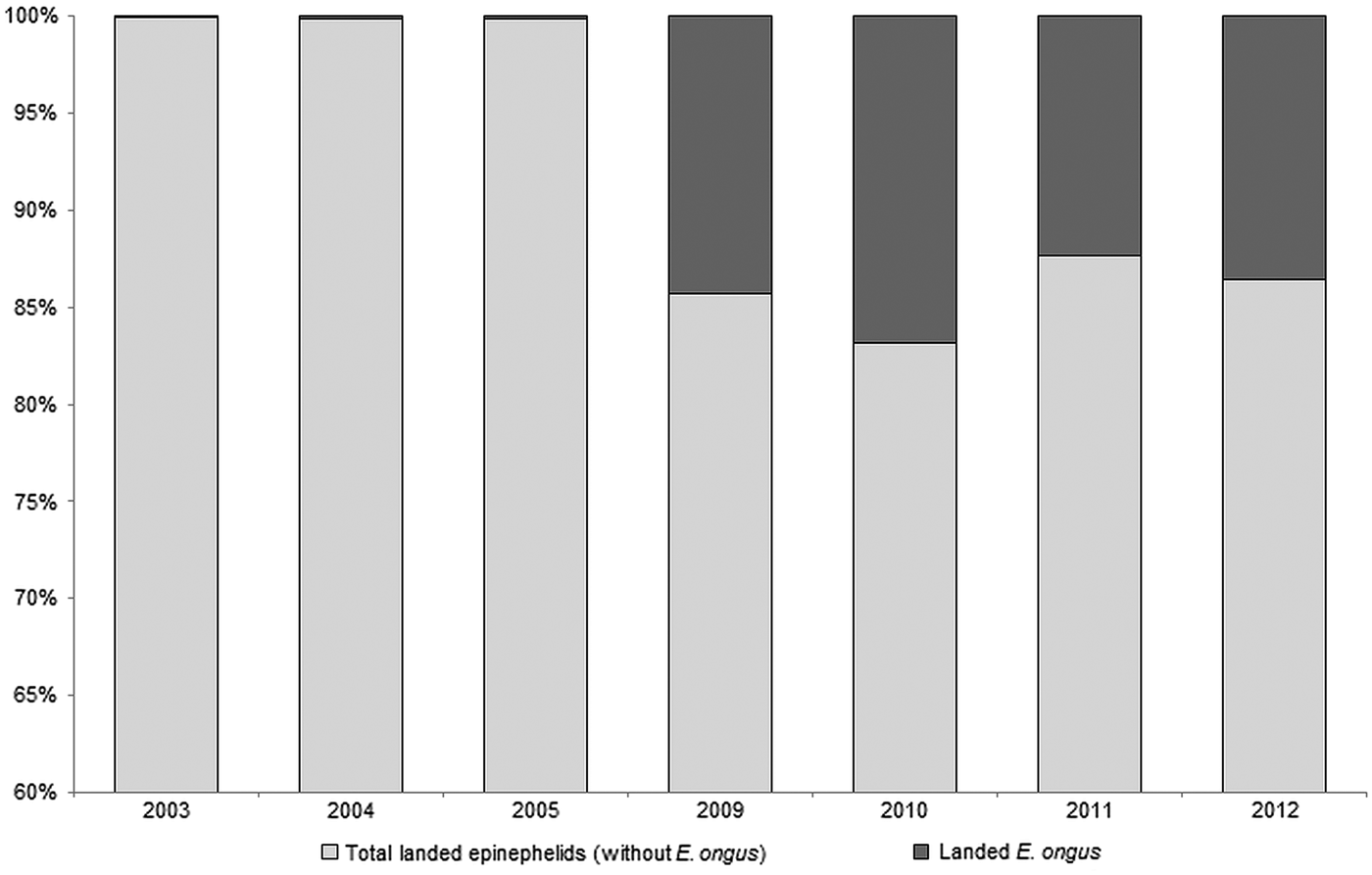
Fig. 1. Development of the contribution of Epinephelus ongus to the total landed epinephelids at National Park Karimunjawa, Indonesia between 2003 and 2012 (based on fish weight)
Karimunjawa is located in the Java Sea, approx. 80 km off the coastal city Jepara, Central Java. This remote archipelago was one of the first areas recognized as being important for the conservation of marine biodiversity in Indonesia (Campbell et al. Reference Campbell, Kartawijaya, Yulianto, Prasetia and Clifton2013). Consequently, Karimunjawa was declared a National Park in 1999 (Campbell et al. Reference Campbell, Kartawijaya, Yulianto, Prasetia and Clifton2013). Nevertheless, fishing is still permitted in Karimunjawa, with exception of certain areas such as spawning aggregation sites (Campbell et al. Reference Campbell, Mukminin, Kartawijaya, Huchery and Cinner2014) and the fishing pressure has distinctly increased during the last decade (Yulianto et al. Reference Yulianto, Hammer, Wiryawan and Palm2015). This resulted in declining stocks of large epinephelids and a shift towards smaller species, especially E. ongus (Fig. 1).
According to Justine et al. (Reference Justine, Beveridge, Boxshall, Bray, Moravec, Trilles and Whittington2010), epinephelids harbour an average of ten different parasite species in the Western Pacific. For example, E. coioides and E. fuscoguttatus harbour 51 and 52 parasite taxa, respectively, in Indonesia alone (Rückert et al. Reference Rückert, Klimpel and Palm2010; Neubert et al. Reference Neubert, Yulianto, Theisen, Kleinertz and Palm2016). In contrast, not a single parasite was documented for E. ongus from Indonesian waters. So far, only three monogeneans, Pseudorhabdosynochus summanae (Young, Reference Young1969) (synonym: Diplectanum summanae Kritsky and Beverley-Burton, Reference Kritsky and Beverley-Burton1986), P. quadratus (Schoelinck and Justine, Reference Schoelinck and Justine2011) and Benedenia fieldsi (Deveney and Whittington, Reference Deveney and Whittington2010) as well as two digeneans, Pearsonellum corventum (Lester and Sewell, Reference Lester and Sewell1989; Overstreet and Køie, Reference Overstreet and Køie1989) and Lepidapedoides angustus (Bray et al. Reference Bray, Cribb and Barker1996), have been recorded for this epinephelid. The present study is the first comprehensive analysis of the parasite fauna of E. ongus worldwide, discussing: (1) the infection pattern, (2) the use of the documented parasite community as environmental indicator and (3) the potential risk of parasite transmission into mariculture systems and vice versa.
MATERIALS AND METHODS
Collection of fish
Samples were taken within the framework of SPICE III – MABICO (Science for the Protection of Indonesian Coastal Marine Ecosystems – Impacts of Marine Pollution on Biodiversity and Coastal Livelihoods). A total of 35 E. ongus were bought from artisanal fishermen collecting live fish in the vicinity of Karimunjawa. Fish were purchased during May and dissected in August 2013. All fish were directly separated into plastic bags, transported on ice and deep frozen (−20 °C) at the Faculty of Fisheries and Marine Sciences, Bogor Agricultural University, Indonesia. Analysed E. ongus had a total length of 25.5 cm (s.e. = 0.4 cm). All available raw data of E. areolatus, E. coioides and E. fuscoguttatus from Indonesian waters (Bali, Java and Sumatra) were used to compare the parasite fauna of E. ongus with commercially important larger epinephelids. In detail, these were 60 E. areolatus with a total length of 32.7 cm (s.e. = 0.3 cm) by Kleinertz (Reference Kleinertz2010), 356 E. coioides with a total length of 28.9 cm (s.e. = 0.3 cm) by Yuniar (Reference Yuniar2005); Rückert (Reference Rückert2006); Kleinertz (Reference Kleinertz2010) and Neubert et al. (Reference Neubert, Yulianto, Theisen, Kleinertz and Palm2016) and 105 E. fuscoguttatus with a total length of 26.8 cm (s.e. = 0.3 cm) by Rückert (Reference Rückert2006).
Parasitological examination
The parasitological investigation was limited on metazoan parasites and followed the standard protocol by Palm (Reference Palm and Mehlhorn2011) and Palm and Bray (Reference Palm and Bray2014). Skin, fins, nostrils, eyes, gills, gill covers, mouth and gill cavity were examined for ectoparasites by using a Zeiss Stemi DV4 binocular microscope. All fluids from the plastic bag in which the fish was frozen were subsequently studied. Examination for endoparasites included the body cavity and mesentery, followed by internal organs, which were separated into Petri dishes and covered with saline solution (0.9%). The microscopic examination of all organs was conducted using a Zeiss Stemi DV4 under 8–32× magnification. A gut wash was performed according to Cribb and Bray (Reference Cribb and Bray2010). The musculature was sliced in thin layers and studied using a transmitting light source. The recorded parasites were transferred to saline solution (0.9%), cleaned, fixed and preserved in 70% ethanol for morphological identification using an Olympus BX53 DIC microscope. The parasites were dehydrated in an ethanol series and transferred to 100% glycerine (Riemann, Reference Riemann, Higgins and Thiel1988). Selected individuals were stained with acetic carmine, dehydrated, cleared with eugenol and mounted in Canada balsam (Palm, Reference Palm2004). According to Paladini et al. (Reference Paladini, Huyse and Shinn2011), Monogenea were treated with proteinase K and mounted in Malmberg's Solution to observe skeletonized structures, which are necessary for species identification. The parasite identification was conducted by using taxonomic keys and original descriptions. For the ectoparasitic monogeneans the literature consulted was provided by Whittington et al. (Reference Whittington, Deveney and Wyborn2001) and Schoelinck and Justine (Reference Schoelinck and Justine2011), for the copepods by Ho and Dojiri (Reference Ho and Dojiri1977); Schmidt and Roberts (Reference Schmidt and Roberts1989); Boxshall and Halsey (Reference Boxshall and Halsey2004) and Ho and Lin (Reference Ho and Lin2004) and for the isopods by Kensley and Schotte (Reference Kensley and Schotte1989). Identification literature of endoparasites was provided by Bray and Cribb (Reference Bray and Cribb1989) for the digeneans, by Palm (Reference Palm2004) for cestodes, and by Anderson et al. (Reference Anderson, Chabaud and Willmott2009); Gibbons (Reference Gibbons2010) and Dewi and Palm (Reference Dewi and Palm2013) for nematodes. In addition, molecular identification of eight Hysterothylacium and one Anisakis specimens was conducted following the protocol by Palm et al. (Reference Palm, Damriyasa, Linda and Oka2008). The sequences of the ITS1, 5.8S and ITS2 rDNA are deposited in GenBank under the accession numbers KU705468 for Hysterothylacium sp. and KU705469 for Anisakis typica.
Quantitative parasite descriptors
The prevalence (P), intensity (I), mean intensity (I m) and mean abundance (A m) of all parasites found were calculated following Bush et al. (Reference Bush, Lafferty, Lotz and Shostak1997). The diversity of the parasite fauna was determined by using the Shannon index of species diversity (Shannon, Reference Shannon1948; Spellerberg and Fedor, Reference Spellerberg and Fedor2003) and the Pielou index of evenness (Pielou, Reference Pielou1966). Furthermore, the Berger–Parker index of dominance was used (Berger and Parker, Reference Berger and Parker1970; May Reference May, Cody and Diamond1975). All indices were calculated for the entire parasite fauna as well as for the endoparasite fauna only. Parasites which were only identified to higher taxonomic levels (such as Nematoda indet.) were omitted from these calculations, because they might represent other recorded taxa. The ecto- to endoparasite ratio was calculated (number of ectoparasite species divided by number of endoparasite species) according to Rückert et al. (Reference Rückert, Hagen, Yuniar and Palm2009a ) (Table 1).
Table 1. Prevalence in % (P), mean intensity (I m), intensity (I) and mean abundance (A m) of parasites from Epinephelus ongus from Karimunjawa, Indonesia, with additional parasitological indices

C, Cestoda; Cr, Crustacea; D, Digenea; M, Monogenea; N, Nematoda.
a Recorded for the first time for E. ongus.
b New locality record (Indonesia).
Data analysis
Raw data by Yuniar (Reference Yuniar2005); Rückert (Reference Rückert2006); Kleinertz (Reference Kleinertz2010) and Neubert et al. (Reference Neubert, Yulianto, Theisen, Kleinertz and Palm2016) (see above) were included in the present study to compare the recorded parasite fauna of E. ongus with previous studied epinephelids from Indonesian waters. Higher taxa as well as records of genera, which were previously identified to the species level were omitted, because they might represent prior recorded species. Statistical analyses were performed using Primer 6 version 6.1.13. To display the level of similarity between all 556 analysed fish, a similarity matrix was constructed applying Bray Curtis similarity measure. Fish without parasites and outliers, defined by unequal results in the Kruskal stress formula, were omitted from analyses. The relation between samples based on the comparison of similarity matrices was displayed by using multi-dimensional scaling (MDS). One-way analyses of similarity were applied to identify the differences in parasite species composition between the epinephelid species (routine ANOSIM, R values close to 1 indicate high differences and close to 0 indicate high similarity between species compositions). SIMPER analysis was applied to test, which parasite species contributed most to the shown differences of analysed epinephelids. All recorded parasites, which could be identified at least to the genus level, were summarized in a parasite host list for Indonesian waters. In the case of one or more given species identification within a genus, further records of this genus were not listed (Table 2).
Table 2. Comparison of the metazoan parasite fauna of Epinephelus areolatus, E. coioides, E. fuscoguttatus and E. ongus from Indonesian coastal waters (+ present, − absent)
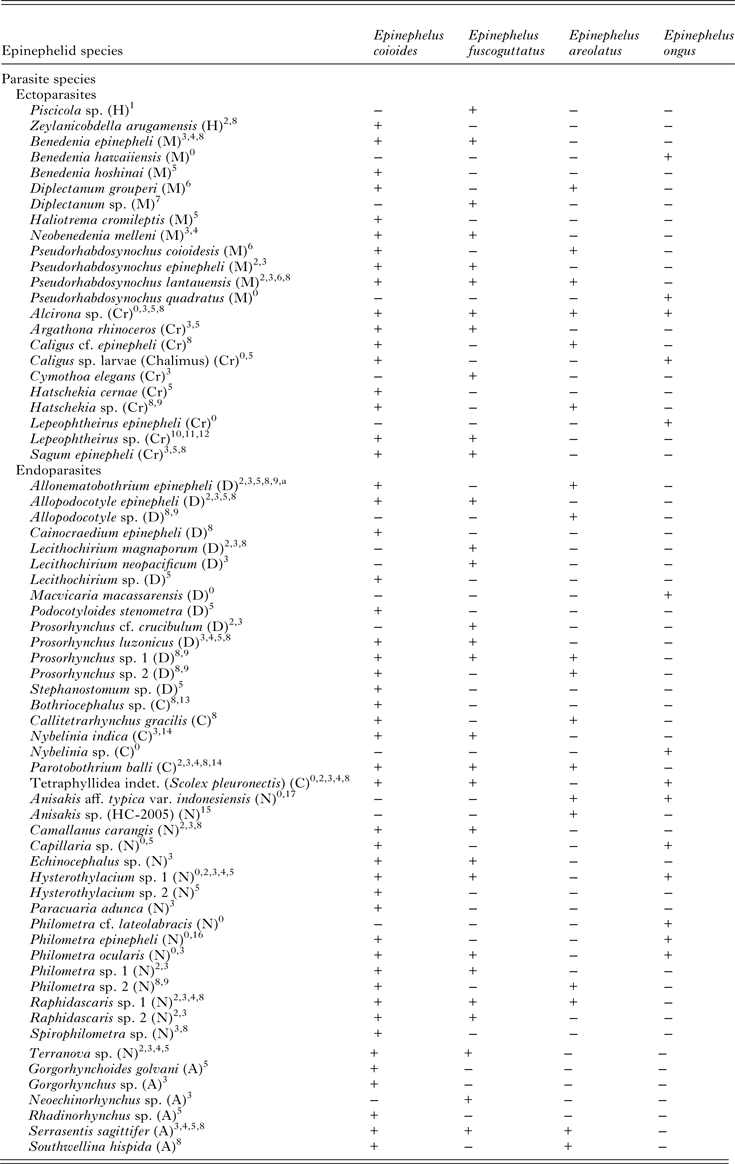
A, Acanthocephala; C, Cestoda; Cr, Crustacea; D, Digenea; H, Hirudinea; M, Monogenea; N, Nematoda.
Source: 0Present study; 1Diani et al. (Reference Diani, Sunyoto and Danakusumah1999); 2Palm and Rückert (Reference Palm and Rückert2009); 3Rückert (Reference Rückert2006); 4Rückert et al. (Reference Rückert, Klimpel, Mehlhorn and Palm2009b ); 5Neubert et al. (Reference Neubert, Yulianto, Theisen, Kleinertz and Palm2016); 6Bu et al. (Reference Bu, Leong, Wong, Woo and Foo1999); 7Wijayati and Djunaidah (Reference Wijayati, Djunaidah, Aliah, Herdis and Surachman2001); 8Kleinertz (Reference Kleinertz2010); 9Kleinertz et al. (Reference Kleinertz, Damriyasa, Hagen, Theisen and Palm2014a ); 10Koesharyani et al. (Reference Koesharyani, Yuasa and Zafran2000); 11Yuasa et al. (Reference Yuasa, Zafran, Koesharyani, Roza and Johnny1998); 12Zafran et al. (Reference Zafran, Roza, Koesharyani, Johnny and Yuasa1998); 13Yuniar (Reference Yuniar2005); 14Palm (Reference Palm2004); 15Palm et al. (Reference Palm, Damriyasa, Linda and Oka2008); 16Dewi and Palm (Reference Dewi and Palm2013); 17Palm et al. (Reference Palm, Theisen, Damriyasa, Kusmintarsih, Oka, Setyowati, Suratma, Wibowo and Kleinertz2016).
a Didymodiclinus sp. in Rückert (Reference Rückert2006); Palm and Rückert (Reference Palm and Rückert2009); Kleinertz (Reference Kleinertz2010); Kleinertz et al. (Reference Kleinertz, Damriyasa, Hagen, Theisen and Palm2014a ).
RESULTS
Parasite community
The examined E. ongus revealed 17 different parasite taxa, seven ecto- and ten endoparasites (ecto- to endoparasite ratio: 0.7). The most speciose parasites were nematodes with seven, followed by crustaceans with four and monogeneans with three species. Less species rich were the cestodes and digeneans with two and one species, respectively. More than 82% (14) of the reported taxa are new host records for E. ongus and four represent first locality records for Indonesian waters (Table 1).
Ectoparasites
The gill infecting monogenean Pseudorhabdosynochus quadratus was the predominating ectoparasite and represents a core species for E. ongus in Karimunjawa (prevalence > 60%). It differs from another recorded Pseudorhabdosynochus species (Pseudorhabdosynochus sp.) by a wider and elongated tube of the skeletonized vagina. The second most prevalent ectoparasite was the copepod Caligus sp. (larval chalimus stage), infecting the gills (second core species). The third most prevalent ectoparasite were gnathiid praniza larvae. These isopods were found on the gills, mouth cavity and operculum. Alcirona sp. (Isopoda), Lepeophtheirus epinepheli (Copepoda) and Benedenia hawaiiensis (Monogenea) were also detected from the gills. Numeric information on the prevalence, intensity, mean intensity and mean abundance of documented ectoparasites are given in Table 1.
Endoparasites
Philometra ocularis as adult females and Hysterothylacium sp. as third stage larvae were the predominating endoparasites. P. ocularis was recorded under the eyes of examined fish, whereas Hysterothylacium sp. occurred in the liver, pyloric caeca, intestine and body cavity. The morphology of Hysterothylacium sp. is similar to Hysterothylacium sp. I as described by Rückert (Reference Rückert2006) and Palm and Rückert (Reference Palm and Rückert2009). The genetic identification of Hysterothylacium sp. revealed highest similarity to H. deardorffoverstreetorum (identity 97%, e.g. GenBank accession number JF730200.1) described from a flounder in Brazil (Knoff et al. Reference Knoff, Felizardo, Iñiguez, Maldonado, Torres, Pinto and Gomes2012). However, the specimens found in this study differed in 24 base pairs of the ITS1, 5.8S and ITS2 rDNA from H. deardorffoverstreetorum, requiring more detailed analyses in terms of species identification. Philometra cf. lateolabracis (Nematoda) was the third most prevalent endoparasite and was recorded from the gonads of female fish. The digenean Macvicaria macassarensis was recorded from the pyloric caeca and intestine and the nematode Capillaria sp. in the stomach of the analysed fish. Beside P. ocularis and Philometra cf. lateolabracis, a third member of Philometridae was found. P. epinepheli was isolated from the tissue under the skin of the opercula. Anisakis typica (Nematoda), which occurred in the stomach, was identified by DNA analysis [identity 100%, GenBank accession number HF911524.1, Kleinertz et al. (Reference Kleinertz, Hermosilla, Ziltener, Kreicker, Hirzmann, Abdel-Ghaffar and Taubert2014b )]. The specimen found in this study is similar to Anisakis sp. 2 by Palm et al. (Reference Palm, Damriyasa, Linda and Oka2008) and A. aff. typica var. indonesiensis Palm et al. (Reference Palm, Theisen, Damriyasa, Kusmintarsih, Oka, Setyowati, Suratma, Wibowo and Kleinertz2016), which is the most frequent genotype of A. typica (sensu lato) in Indonesian waters. A nematode which could not be identified to a precise taxonomic level due to its poor condition was found in the stomach, intestine and pyloric caeca (Nematoda indet.). The larval trypanorhynch cestode Nybelinia sp. with inverted tentacles was recorded from the pyloric caeca, and larval tetraphyllids named as Tetraphyllidea indet. (Scolex pleuronectis), from the intestine of the analysed E. ongus. Numeric information on the prevalence, intensity, mean intensity and mean abundance of the recorded endoparasites are given in Table 1.
Parasitological indices
The Shannon index of species diversity for E. ongus reached 0.17. If only endoparasites were considered, the Shannon index of species diversity differed distinctly and reached a more than ten times higher value of 1.93. This is the result of the predominating ectoparasite P. quadratus, expressed by a Berger–Parker index of dominance of 0.97. If only endoparasites were considered the Berger–Parker index of dominance decreased distinctly to 0.27. A similar pattern was recognised for the Pielou index of evenness with values of 0.06 for the entire parasite fauna and 0.84 for the endoparasite fauna (Table 1).
Comparison of analysed epinephelids
A total of 66 different parasite species, excluding species identified to higher taxonomic levels, represent the parasite fauna of the four considered epinephelid species in Indonesian waters (Table 2). MDS revealed a distinctly different parasite infection pattern for E. ongus, E. areolatus and E. fuscoguttatus whereas E. coioides is not clearly separating from the first three epinephelids (Fig. 2). The ANOSIM significantly demonstrated that the difference between the parasite composition of all four epinephelid species is not distinctive (Global R: 0.30, P < 0.01). The pair-by-pair comparisons showed that E. coioides is responsible for this finding (E. coioides vs E. areolatus (R: 0.30, P < 0.01), E. coioides vs E. fuscoguttatus (R: 0.18, P < 0.01) and E. coioides vs E. ongus (R: 0.36, P < 0.01)). The remaining three epinephelids E. ongus, E. areolatus and E. fuscoguttatus demonstrated a high separation based on their parasites [E. ongus vs E. areolatus (R: 0.90, P < 0.01), E. ongus vs E. fuscoguttatus (R: 0.95, P < 0.01), E. areolatus vs E. fuscoguttatus (R: 0.94, P < 0.01)]. The SIMPER analysis revealed that P. quadratus, Hysterothylacium sp. 1 and P. ocularis are the main contributors separating E. ongus from the remaining three epinephelids. Allonematobothrium epinepheli, Anisakis aff. typica var. indonesiensis (Palm et al. Reference Palm, Theisen, Damriyasa, Kusmintarsih, Oka, Setyowati, Suratma, Wibowo and Kleinertz2016) and Hatschekia sp. contributed most to the separation of E. areolatus and Alcirona sp., All. epinepheli and Raphidascaris sp. to the separation of E. fuscoguttatus. Pseudorhabdosynochus lantauensis, Prosorhynchus luzonicus and Alcirona sp. are responsible for the minor separation of E. coioides from the remaining three epinephelid species.

Fig. 2. Multidimensional scaling plot of the parasite fauna from Epinephelus areolatus
![]() , E. coioides
, E. coioides
![]() , E. fuscoguttatus
, E. fuscoguttatus
![]() and E. ongus
and E. ongus
![]() in Indonesian waters based on Bray Curtis similarity
in Indonesian waters based on Bray Curtis similarity
DISCUSSION
The information on the parasite fauna of E. ongus is very limited. So far, only five species have been recorded (see the ‘Introduction’), including the records of Benedenia fieldsi (Deveney and Whittington, Reference Deveney and Whittington2010) and Pseudorhabdosynochus summanae (Young, Reference Young1969), which must be considered more or less as questionable. B. fieldsi was isolated from an aquarium fish and a possible transfer from another fish species in the same fish tank cannot be excluded. P. summanae was originally described from Epinephelus summana sampled in Australia (Young, Reference Young1969), but E. summana is endemic to the Red Sea, Gulf of Aden and Socotra Yemen (Heemstra and Randall, Reference Heemstra and Randall1993; Craig et al. Reference Craig, Sadovy de Mitcheson and Heemstra2011). Consequently, Justine (Reference Justine2007) already concluded that E. summana cannot be the type-host for this monogenean and declared E. ongus or E. coeruleopunctatus as potential type-host. Schoelinck and Justine (Reference Schoelinck and Justine2011) suggested that E. ongus was the original type-host of P. summanae, however, not considering Heemstra and Randall (Reference Heemstra and Randall1993) who stated that additional white spotted groupers such as E. corallicola, a species which is also known from Australia (Froese and Pauly, Reference Froese and Pauly2015), are often confused with E. summana. However, Lester and Sewell (Reference Lester and Sewell1989) reported Diplectanum summanae from E. ongus sampled at Heron Island, Australia. D. summanae is a synonymised name of P. summanae. Therefore, we agree with Schoelinck and Justine (Reference Schoelinck and Justine2011) which nominated E. ongus as type-host of P. summanae. Considering the small number of recorded parasites as well as the uncertainty by one of five records, the present study gives a first comprehensive insight into the parasite fauna of E. ongus.
Almost all recorded taxa are new host records for E. ongus. It is very interesting that only four of 17 taxa represent new locality records, although E. ongus was never parasitologically sampled in Indonesia before. Three of these four previously unknown taxa are ectoparasites (B. hawaiiensis, L. epinepheli and P. quadratus) and only one belongs to the endoparasites (Philometra cf. lateolabracis). Furthermore, the previously recorded endoparasites are known from other epinephelids of Indonesian waters (E. areolatus, E. coioides and E. fuscoguttatus) (Table 2). The only exception is M. macassarensis, which was originally found in a lethrinid from Sulawesi by Yamaguti (Reference Yamaguti1952). Thus, the endoparasitic fauna of E. ongus was distinctly dominated by generalist parasites, which are already known for epinephelids from Indonesian waters (Palm and Rückert, Reference Palm and Rückert2009; Rückert et al. Reference Rückert, Klimpel and Palm2010; Kleinertz et al. Reference Kleinertz, Damriyasa, Hagen, Theisen and Palm2014a ; Kleinertz and Palm, Reference Kleinertz and Palm2015; Neubert et al. Reference Neubert, Yulianto, Theisen, Kleinertz and Palm2016). The most abundant endoparasitic taxon was the Nematoda, which contributed 67% to all recorded endoparasites of E. ongus. This is the main difference to previously studied epinephelids, where the nematodes hold between 25 and 33% of the entire endoparasite records (Palm and Rückert, Reference Palm and Rückert2009; Rückert et al. Reference Rückert, Klimpel and Palm2010; Kleinertz et al. Reference Kleinertz, Damriyasa, Hagen, Theisen and Palm2014a ; Kleinertz and Palm, Reference Kleinertz and Palm2015; Neubert et al. Reference Neubert, Yulianto, Theisen, Kleinertz and Palm2016). Indonesia is the most diverse marine region in the world (Allen, Reference Allen2008). The fish analysed in this study were obtained from one of the most remote archipelagos in this tropical diversity hotspot. Consequently, the recorded parasite fauna represents the most common parasites of E. ongus in an overfished (Yulianto et al. Reference Yulianto, Hammer, Wiryawan and Palm2015), but environmental less affected habitat. It is evident that the parasite richness of E. ongus does not reach the species numbers that were recorded for previously investigated E. coioides and E. fuscoguttatus in Indonesia. However, the records reach a similar level as reported for E. areolatus (Table 2). The parasite composition of analysed E. areolatus, E. fuscoguttatus and E. ongus differs significantly, associated with an overarching pattern for E. coioides (Fig. 2). This finding as well as the predominance of nematodes is remarkable, because the ecology, behaviour and feeding of these species have been reported as approximately the same (Heemstra and Randall, Reference Heemstra and Randall1993; Craig et al. Reference Craig, Sadovy de Mitcheson and Heemstra2011). However, one striking difference can be found in the maximum size of these four epinephelids. E. coioides and E. fuscoguttatus reach a total length up to 120 cm, whereas the maximum recorded length of E. ongus and E. areolatus is about 40 cm (Heemstra and Randall, Reference Heemstra and Randall1993; Craig et al. Reference Craig, Sadovy de Mitcheson and Heemstra2011). The analysed fish specimens in this study ranged between 19.5 and 46.4 cm. Thus, most E. coioides and E. fuscoguttatus were juveniles (Rückert, Reference Rückert2006; Kleinertz, Reference Kleinertz2010), whereas all E. areolatus and E. ongus were adults (for E. areolatus, see Kleinertz, Reference Kleinertz2010). Juvenile E. coioides prefer sand, mud and gravel, while juvenile E. fuscoguttatus are often found in seagrass areas (Heemstra and Randall, Reference Heemstra and Randall1993). Adult E. areolatus are likewise found over seagrass or on fine sediment bottoms, but in deeper areas (Carpenter and Niem, Reference Carpenter and Niem1999). E. ongus typically occurs in coral reef habitats and on rocky bottoms (Craig et al. Reference Craig, Sadovy de Mitcheson and Heemstra2011). Thus, the considered epinephelids prefer different habitats within the analysed size range. This contributes to the recorded different parasite composition (Fig. 2). However, it is remarkable that also generalist parasites, like Alcirona sp., All. epinepheli, Hysterothylacium sp. 1 and P. ocularis, contribute to the differentiation of the analysed fishes. Even if these parasites can use a broad range of hosts, a pattern can be observed, which allows separation of analysed epinephelids (Fig. 2).
Rückert et al. (Reference Rückert, Klimpel, Mehlhorn and Palm2009b ); Rückert et al. (Reference Rückert, Klimpel and Palm2010) and Palm et al. (Reference Palm, Yulianto, Theisen, Rückert and Kleinertz2015) reported the potential risk of parasite transmission between cultured and wild epinephelids. Due to the high number of recorded generalist parasites (see above), this is also the case for E. ongus. If one of the recorded parasite species has the potential to increase mortality, decrease fish health or product quality, is a matter of further investigations. This might have relevance in future mariculture development in Indonesia. Parasites infecting several of the four analysed epinephelid species (Fig. 2) contribute most to this potential risk, and can be easily introduced to new localities through the establishment of new mariculture facilities or stocking with non-native species. Karimunjawa is one of the remotest islands of Indonesia. However, mariculture activities are growing in the archipelago (Campbell et al. Reference Campbell, Kartawijaya, Prasetia and Pardede2010). The present study on E. ongus might serve as a reference in terms of: (1) future monitoring programs of these activities (Palm et al. Reference Palm, Kleinertz and Rückert2011) as well as (2) environmental indication based on fish parasites (Kleinertz and Palm, Reference Kleinertz and Palm2015; Neubert et al. Reference Neubert, Yulianto, Theisen, Kleinertz and Palm2016). We suggest, that E. ongus is a suitable species for this purposes due to its increasing contribution to fish landings as well as the decreasing abundance of previously used larger epinephelids like E. coioides. Recently, the water quality in Karimunjawa was defined as very good with low, spatial limited inputs of domestic sewage (Sugianti and Mujiyanto, Reference Sugianti and Mujiyanto2014). However, also in Karimunjawa water pollution has increased in recent years and it can be expected that the anthropogenic impact will increase at the same rate as the coastal development increases in Karimunjawa (Campbell et al. Reference Campbell, Kartawijaya, Yulianto, Prasetia and Clifton2013).
Ectoparasitic flukes have direct life cycles without intermediate hosts (Whittington, Reference Whittington and Rohde2005). Under polluted conditions increasing infestation rates, high individual numbers and an unequal distribution in favour of ectoparasites were often reported (e.g. Haensly et al. Reference Haensly, Neff, Sharp, Morris, Bedgood and Boem1982; Skinner, Reference Skinner1982; Khan and Kiceniuk, Reference Khan and Kiceniuk1988; Marcogliese and Cone, Reference Marcogliese and Cone1996; MacKenzie, Reference MacKenzie1999; Dzikowski et al. Reference Dzikowski, Paperna and Diamant2003). The parasite fauna of E. ongus at Karimunjawa was predominated by the diplectanid monogenean P. quadratus expressed by a Berger–Parker index of dominance of 0.97 and a Pielou index of evenness of 0.06 (Table 1). The mean intensity of 260.1 is the highest ever recorded for the genus Pseudorhabdosynochus from free-living epinephelids in Indonesia (Palm and Rückert, Reference Palm and Rückert2009; Rückert et al. Reference Rückert, Klimpel and Palm2010; Kleinertz et al. Reference Kleinertz, Damriyasa, Hagen, Theisen and Palm2014a ; Kleinertz and Palm, Reference Kleinertz and Palm2015; Neubert et al. Reference Neubert, Yulianto, Theisen, Kleinertz and Palm2016). For a near natural environment, like Karimunjawa, this was previously unknown. This finding might be explained with the age of sampled fish. In contrast to E. ongus, the previous studied E. coioides and E. fuscoguttatus were juveniles (see above). Therefore, E. ongus were substantially older in the analysed size range and it appears that the relatively old E. ongus accumulated P. quadratus over time. In addition, it seems that P. quadratus dominated the copepod Caligus sp. in quantity of infestation. Caligus sp. was found at the same site on the gills with a high prevalence (60.0%), but with a distinctly lower mean intensity of 3.0, although Caligus sp. is known to occur with high individual numbers (Neubert et al. Reference Neubert, Yulianto, Theisen, Kleinertz and Palm2016). Another interpretation of the massive Pseudorhabdosynochus infection is that the environmental conditions off Karimunjawa are not as good as reported by Sugianti and Mujiyanto (Reference Sugianti and Mujiyanto2014). This would be coherent with the low Shannon index of species diversity. However, if only the endoparasites were considered, the parasite fauna of E. ongus must be assessed as highly diverse, as the Shannon index of species diversity reached 1.93. This is the highest value documented for an epinephelid in Indonesian waters (see above). For comparison, the highest recorded Shannon index of species diversity of endoparasites for E. coioides reached 1.84, for E. fuscoguttatus 1.78 and for E. areolatus 1.61, and these values are from unaffected habitats as well (Rückert, Reference Rückert2006; Palm and Rückert, Reference Palm and Rückert2009; Rückert et al. Reference Rückert, Klimpel and Palm2010; Palm et al. Reference Palm, Kleinertz and Rückert2011; Kleinertz et al. Reference Kleinertz, Damriyasa, Hagen, Theisen and Palm2014a ; Kleinertz and Palm, Reference Kleinertz and Palm2015; Neubert et al. Reference Neubert, Yulianto, Theisen, Kleinertz and Palm2016). The Shannon index of species diversity is known to indicate diversity loss of endoparasites in affected environments (Rückert, Reference Rückert2006; Rückert et al. Reference Rückert, Hagen, Yuniar and Palm2009a ). Referring to the highly diverse endoparasite fauna of E. ongus, the environmental conditions in Karimunjawa must be considered as fairly natural. Currently, the marine food web appears to be unspoiled, enabling many endoparasitic species to complete their complex life cycles. The endoparasitic fauna is normally distributed, depicted by a Berger–Parker index of dominance of 0.27 and a Pielou index of evenness of 0.84. However, it is interesting that only a single digenean species was found (see Table 1), although epinephelids appear to harbour rich assemblages of digeneans (Cribb et al. Reference Cribb, Bray, Wright and Pichelin2002). A diverse endoparasite fauna is a hallmark of unpolluted environments (e.g. Lafferty, Reference Lafferty1997; MacKenzie, Reference MacKenzie1999). Consequently, the high number of recorded nematode species indicates that the marine ecosystem off Karimunjawa is healthy and provides the manifold intermediate host fauna, which is needed to fulfil the multiple host life cycles of these parasites. This is underlined by the recorded cestodes, which are indicators of good environmental conditions as well (Palm, Reference Palm and Mehlhorn2011). According to Rückert et al. (Reference Rückert, Hagen, Yuniar and Palm2009a ), the documented ecto- to endoparasite ratio of 0.7 classifies Karimunjawa likewise as habitat with natural conditions. However, it should be kept in mind, that on the one hand the massive infection with Pseudorhabdosynochus and the low digenean diversity is not characteristic for epinephelids from nearly unaffected habitats such as Karimunjawa Islands (Kleinertz et al. Reference Kleinertz, Damriyasa, Hagen, Theisen and Palm2014a ; Kleinertz and Palm, Reference Kleinertz and Palm2015); and on the other hand that this is the first comprehensive study on the parasites of E. ongus. Thus, the findings are restricted to 35 specimens from one locality, which makes further parasitological investigations on this significant fish urgently needed.
ACKNOWLEDGEMENTS
We are thankful for institutional support to the Leibniz Center for Tropical Marine Ecology, GmbH, Germany, and the Bogor Agricultural University (IPB), Indonesia. Special thanks to Dr Am Azbas Taurusman from IPB for his personal initiative, providing laboratory space and organizational support during laboratory work. We would also like to thank the National Park authority for the sampling permit (approval number: 18/BA/BTNKJ-3/2013).
FINANCIAL SUPPORT
Financial support was provided by the German Federal Ministry of Education and Research (grant number 03F0641D) within the framework of the joint Indonesian–German research project SPICE III – MABICO project (Science for the Protection of Indonesian Coastal marine Ecosystems – Impacts of marine pollution on biodiversity and coastal livelihoods).
CONFLICT OF INTEREST
None.






