INTRODUCTION
Pediculosis (infestation with head lice) has been reported as a chronic public health problem worldwide, particularly in children aged 3–13 (Speare and Buettner, Reference Speare and Buettner1999; Diamantis et al. Reference Diamantis, Morrell and Burkhart2009; Currie et al. Reference Currie, Reynolds, Glasgow and Bowden2010). Therapeutic management of pediculosis is challenging with increasing worldwide prevalence due to product failures resulting from resistance, incorrect application, formulation changes and misdiagnosis (Toloza et al. Reference Toloza, Lucia, Zerba, Masuh and Picollo2008). With a limited number of effective pediculicides available, plant-derived compounds hold great potential due to demonstrated repellent, ovicidal, adulticidal and feeding inhibition activity against various arthropods including head lice (Audino et al. Reference Audino, Vassena, Zerba and Picollo2007).
Like tea tree oil (TTO), kunzea oil (KO) is a myrtaceous essential oil; although it differs in composition to TTO, comprising about 70% monoterpenes but with characteristic sesquiterpenes (Thomas et al. Reference Thomas, Narkowicz, Jacobson and Davies2010). Kunzea spp. plant extracts have insecticidal properties (Khambay et al. Reference Khambay, Beddie, Simmonds and Green1999, Reference Khambay, Beddie and Simmonds2002). KO was shown to be repellent to host-seeking mosquitoes in the laboratory testing with comparable efficacy to citronella (Thomas et al. Reference Thomas, Narkowicz, Peterson, Jacobson and Narayana2009a , Reference Thomas, Webb, Narkowicz, Jacobson, Peterson, Davies and Russell b ). Given the demonstrated repellent activity against mosquitoes, it has been suggested that similar effectiveness may be achieved against other haematophagous arthropods. The aim of this study was to investigate the ability of commercially available KO for its pediculicidal properties.
EXPERIMENTAL METHODS
Collection of head lice for laboratory studies
This work was approved by the University of South Australia's Human Research Ethics Committee (approvals 30059 and 32538). Head lice were obtained directly from the children (following written parental consent) at two primary schools in South Australia as well as a purpose run head lice clinic at the University of South Australia. Head lice were removed from individuals by combing dry hair from scalp to tip using a head lice comb (Barker and Altman, Reference Barker and Altman2010). Head lice were removed and placed into a Petridish for holding (maximum 15 min) until the testing could commence.
Pediculicidal assay
Each test Petridish contained a filter paper (7 cm diameter) that was uniformly covered in 1 mL treatment or control products. These amounts were pre-measured before trials and then applied immediately prior to testing against lice. KO (Therapeutic Goods Administration ARTG entry 72143; Ducane Estate, Waterhouse, Tasmania), both a 100 and 5% solution [in ethanol (90% of 70% v/v ethanol and 5% glycerol)], was tested along with two commercially available formulations, TTO head lice gel (Integria Healthcare Australia Pty Ltd, Eight Mile Plains, QLD; containing 5% TTO) and permethrin spray (Garrards Pesticides, Clovelly Park SA; containing 500 g kg−1 permethrin). The permethrin was included here as a positive control insecticide. Dimethicone-free hair conditioner (Moogoo Skin Care Pty Ltd, Brisbane, QLD) was tested as a wet control that may possess a suffocant effect against lice but no toxicosis.
Each formulation was applied evenly onto the filter paper contained within a Petridish and allowed it to dry for approximately 1 h prior to testing. As only head lice removed from a volunteer within 15 min were used, for each replicate test, 5–10 head lice were used. Head lice were transferred to a dish using a small, soft, bristled paintbrush and placed within the centre of the treated filter paper. Head lice were observed every 15 min for up to 120 min and the number of dead individual lice recorded at each time point. The number of replicate tests for each formulation and control varied between 3 and 9. Moribund lice responsive to touch with a probe were considered alive, while death was determined to have occurred when there was no response to the probe. Lice classified as dead never recovered. Mortality of head lice was recorded as the mean number of dead head lice across the replicate tests. Kaplan–Meier survival analysis (STATA version 12, College Station, USA) was used to compare pediculicidal effects of different treatments and to calculate mean survival times.
RESULTS
Exposure to KO, both as a 5% and neat oil (100%, undiluted) resulted in 100% mortality within 120 min (Fig. 1) with the mean survival times of 17·1 min (5% solution) and 34·8 min (neat oil; 100%, undiluted) (Table 1). The mean survival time for the 5% KO was the shortest of any other formulation tested and has now been shown to be extremely effective at killing lice in vitro and demonstrated the similar effectiveness to TTO (Fig. 1; Table 1). Kaplan–Meier survival analysis showed no significant difference in the mortality rate of KO compared with the TTO-based formulation (log-rank test, P > 0·05), which is also reflected in the mean survival times of lice exposed to the two oils (Table 1). Interestingly, the mean survival times of the permethrin-based formulation were substantially longer than both the KO and TTO-based formulations (Table 1). Over 40% mortality was recorded in head lice exposed to the plain hair conditioner after 90 min, a significantly lower rate (log-rank test, P < 0·001) than any of the botanical or insecticide-based products. Less than 10% mortality was recorded in head lice in the dry control (nothing added to the filter paper) category after 120 min (Fig. 1). These differences in the mortality between KO/TTO and the dry and wet controls were statistically significant (log-rank test, P < 0·001). Survival analysis also revealed no significant differences in the mortality between KO and TTO.
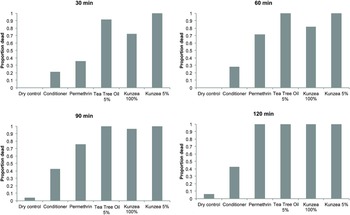
Fig. 1. Pediculicidal properties of kunzea oil preparations compared with positive and negative controls.
Table 1. Mean individual louse survival times and 95% confidence intervals (CI) when exposed to in vitro pediculicidal treatments, in descending order of pediculicidal activity

DISCUSSION
This in vitro study demonstrates that the mean mortality of head lice exposed to KO does not differ appreciably to those exposed to currently registered formulations containing TTO. With the perception that formulations containing synthetic insecticides such as permethrin (Durand et al. Reference Durand, Bouvresse, Berdjane, Izri, Chosidow and Clark2012) may be driving the development of insecticide resistance, there is growing interest in novel pediculicides, particularly those of botanical origin, and a partial summary of the key candidates is summarized in Table 2. Botanical extracts avoid the problems arising from residual effects seen with synthetic pediculicides (Webb and Russell, Reference Webb and Russell2011) and are very unlikely to yield resistance in lice because of multiple modes of action of each of their active principles (Carpinella et al. Reference Carpinella, Miranda, Almirón, Ferrayoli, Almeida and Palacios2007). While commercial products containing botanical extracts are widely available and commonly used as both pediculicides and repellents against head lice, TTO-based formulations are most common. The results of this study demonstrate that KO as an active ingredient will provide comparable control of head lice to that of TTO.
Table 2. A partial summary of various essential oil with documented activity against Pediculus humanus capitis

Adopted from Rossini et al. (Reference Rossini, Castillo and González2008) and Yang et al. (Reference Yang, Lee, Clark and Ahn2004).
Given the interest amongst the community for ‘natural’ solutions to head lice, it is important that appropriate testing be conducted to confirm the efficacy of any new pediculicide formulations (Heukelbach et al. Reference Heukelbach, Canyon, Oliveira, Muller and Speare2008). Based on the results of this study, there is great potential in KO and that it has already demonstrated efficacy above the 80% mortality threshold suggested (Heukelbach et al. Reference Heukelbach, Canyon, Oliveira, Muller and Speare2008). However, while in vitro testing shows great potential, it will be critical that controlled field trials and therapeutic potential of formulated products be assessed (Burkhart and Burkhart, Reference Burkhart and Burkhart2001). The need for understanding the synergistic effect of non-active ingredients in commercial formulations was highlighted by the results of the testing presented here with 5% KO recording a lower mean survival time compared with the neat oil (100%, undiluted).
Many local health authorities continue to preference the ‘wet comb technique’ as a head lice management strategy to reduce the possibility of overexposure to insecticides and minimizing the risk of insecticide resistance (Maxwell et al. Reference Maxwell, Crawford and Rose2014). However, the results of this study indicated that the use of conditioner does not provide the same level of pediculicidal action as that provided by KO. The dimethicone-free hair conditioner was tested but our data indicate that hair conditioner can kill only some adult lice, presumably through a suffocation effect. The use of hair conditioner without any active pediculicides present, physical suffocant properties of head lice will only assist in treatment if combined with a thorough combing and removal of ‘stunned’ head lice.
KO appears to be safe for topical application on the skin. KO has been tolerated well in clinical studies (human and animal) with concentrations ranging from 10 to 100% with no major documented solicited localized and/or systemic adverse drug reactions (Jacobson et al. Reference Jacobson, Narkowicz, Thomas and Peterson2009; Thomas et al. Reference Thomas, Narkowicz, Peterson, Jacobson and Narayana2009a , Reference Thomas, Webb, Narkowicz, Jacobson, Peterson, Davies and Russell b , Reference Thomas, Jacobson, Narkowicz and Peterson2015). This includes application of oil and/or formulations at skin sites including open wounds (animal trial), inflamed skin (e.g. scalp psoriasis) and nails infected with fungal infection (both human trials). However, more work is required to further establish the optimum safe concentration of the oil for topical application. Repeated analyses of aged KO (6–36 months, stored at room temperature) did not show any noticeable change in the chemical profile of the oil indicating little degradation of oil resulting from oxidation and polymerization (J. Thomas, unpublished data). KO has been recently examined for its activity against ectoparasites (scabies mites and head lice) and did not show any appreciable activity against scabies mites below 5% (J. Thomas, 2015, unpublished data). The 5% TTO has demonstrated remarkable activity against human scabies mites (Walton et al. Reference Walton, McKinnon, Pizzutto, Dougall, Williams and Currie2004), and the commercial 5% TTO gel formulation has been widely used for the management of head lice in Australia. Therefore, a 5% KO formulation has been used in our study for a direct comparison with the proprietary 5% TTO head lice gel.
This study confirms the pediculicidal potential of KO and further investigation is required to determine formulations that can be used as effective alternatives to botanical or synthetic insecticides for the management of ectoparasitic infestations in humans and animals. Despite active promotion of the head lice treatments, studies have found carers vary greatly in the treatments used against pediculosis (Maxwell et al. Reference Maxwell, Crawford and Rose2014). Recommendations for pediculicide use should be regularly reviewed in light of developing resistance levels and new therapies. The ideal head lice treatment has been defined as being safe, effective, easy to use, inexpensive and available without a prescription and as a single dose (Frankowski and Bocchini, Reference Frankowski and Bocchini2010). Regardless of the development of novel pediculicide formulations, community education will remain a critical component of head lice management strategies.
ACKNOWLEDGEMENTS
Louise Massie provided great assistance at the Sansom Institute for Health Research Clinical Trials Facility. The cooperation of primary schools and their Principals was pivotal to this study and greatly appreciated.
FINANCIAL SUPPORT
This study was funded by the Faculty of Health, University of Canberra internal grant awarded to Dr Jackson Thomas.
CONFLICT OF INTEREST
The authors have no competing interests.
ETHICAL STANDARDS
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. This work was approved by the University of South Australia's Human Research Ethics Committee (approvals 30059 and 32538).





