Introduction
Our understanding and interpretation of theropod dinosaurs has progressed from originally reptilian to increasingly more avian. Many features we previously thought unique to modern birds have now been found in their dinosaur ancestors (Chiappe Reference Chiappe2009). However, behavioral interpretations remain challenging—principal among these are nesting behaviors. Currently there is no consensus on whether maniraptoran theropod dinosaurs employed thermoregulatory contact incubation, passive incubation, or simply nest guarding (Varricchio et al. Reference Varricchio, Jackson and Trueman1999; Grellet-Tinner et al. Reference Grellet-Tinner, Chiappe, Currie, Koppelhus, Shugar, Wright, Currie, Koppelhus, Shugar and Wright2004). Perhaps all of these behaviors were present in one group of theropods or another, but it is not clear for oviraptorids and troodontids—the groups with the most nest-related fossils. These fossils include adult material associated with eggs (Norell et al. Reference Norell, Clark, Chiappe and Dashzeveg1995, Reference Norell, Balanoff, Barta and Erickson2018; Varricchio et al. Reference Varricchio, Jackson, Borkowski and Horner1997; Fanti et al. Reference Fanti, Currie and Badamgarav2012) as well as embryonic remains (Norell et al. Reference Norell, Clark, Demberelyin, Rhinchen, Chiappe, Davidson and Novacek1994, Reference Norell, Clark and Chiappe2001; Varricchio et al. Reference Varricchio, Horner and Jackson2002). Some authors argue that evidence suggests parents were engaged in active incubation (Varricchio et al. Reference Varricchio, Jackson, Jackson and Zelenitsky2013, Reference Varricchio, Kundrat and Hogan2018). Others are dubious of such behavior, and believe that nest guarding of buried eggs was more likely (Wesołowski Reference Wesołowski2004; Deeming Reference Deeming2006; Jones and Geist Reference Jones, Geist, Brett-Surman, Holtz, Farlow and Walters2012; Yang et al. Reference Yang, Wiemann, Xu, Cheng, Wu and Sander2019b).
For Troodon in particular, one major criticism of contact incubation is the presumed inefficiency of incubating partially buried eggs (Ruben et al. Reference Ruben, Jones and Geist2003). Several fossilized egg clutches have been linked with Troodon formosus (Varricchio et al. Reference Varricchio, Jackson and Trueman1999, Reference Varricchio, Horner and Jackson2002) and nest attendance behavior (Varricchio et al. Reference Varricchio, Jackson, Borkowski and Horner1997). These Troodon nest sites consistently feature eggs that would have been partially buried in life (Horner and Weishampel Reference Horner and Weishampel1988; Varricchio et al. Reference Varricchio, Jackson, Borkowski and Horner1997). Partial burial is further supported by analysis of eggshell porosity that shows differing pore densities along the length of the egg (Varricchio et al. Reference Varricchio, Jackson, Jackson and Zelenitsky2013). Many modern reptiles and some modern birds incubate their eggs using energy generated from mounds of decaying vegetation, and although this has been suggested as a possibility for Troodon, there is no geologic evidence for plant material in the nest (Varricchio et al. Reference Varricchio, Jackson and Trueman1999). Partial burial of eggs is rarely practiced by extant vertebrates. The most well-known example is the Egyptian plover (Pluvianus aegyptius) (Grellet-Tinner et al. Reference Grellet-Tinner, Chiappe, Norell and Bottjer2006); though this may not be an appropriate comparison. The Egyptian plover inhabits hot, dry African climates where it exhibits the specialized behavior of egg thermoregulation via evaporative cooling. It has been suggested that perhaps Troodon engaged in similar behavior (Grellet-Tinner et al. Reference Grellet-Tinner, Chiappe, Norell and Bottjer2006), but in dinosaurs partially buried nests may represent intermediate behavior instead. After all, if complex behavior can evolve incrementally through generations (Lorenz Reference Lorenz1958; Gould Reference Gould1982; Gomez and Miikkulainen Reference Gomez and Miikkulainen1997), then an ancestor of modern birds likely did use partial-burial nesting strategies (as ancestral reptiles used subterranean nests and modern birds tend to build subaerial ones) (Varricchio and Jackson Reference Varricchio and Jackson2016). Still, the question remains as to whether or not these partially buried nests coincided with contact incubation.
Because questions about the inefficiency of partially buried eggs cannot be satisfactorily addressed by comparisons to modern reptiles and birds or through strict geologic data, an actualistic study was devised to examine the viability of the behavior, with regard to energy efficiency, in both an indoor controlled mock-nesting environment and an outdoor system. Models can serve as appropriate placeholders when past systems differ significantly from what can be observed in modern organisms. Although approximations of past behavior or physiology will always suffer from assumptions, such models still provide researchers with a foothold for further research. These investigations have become an increasingly useful way for paleontologists to explore prehistoric processes. Some examples include assessments of the airflow and thermoregulation regarding the plates of Stegosaurus (Farlow et al. Reference Farlow, Thompson and Rosner1976), the sail of Edaphosaurus (Bennett Reference Bennett1996), and Microraptor flight capabilities (Alexander et al. Reference Alexander, Gong, Martin, Burnham and Falk2010; Dyke et al. Reference Dyke, de Kat, Palmer, van der Kindere, Naish and Ganapathisubramani2013; Palmer Reference Palmer2014).
This purpose of this experiment is to investigate whether or not incubating partially buried eggs through contact is efficient enough that the two behaviors could have coincided. For the purpose of this study, inefficiency constitutes a system where energy input from the adult is so high as to render the feat infeasible, or energy leaches from the eggs to the sediment at a rate great enough to hinder the eggs’ capacity to consistently maintain temperatures above that of the air or soil. So, an inefficient system is one where an adult sitting on the eggs confers no thermodynamic advantage (i.e., warmer or more regular temperatures) compared with non-incubated subaerial or non-incubated shallow subterranean nest states. Temperature plays a critical role in the duration of egg-bound embryo development (Szczerbińska et al. Reference Szczerbińska, Majewska, Tarasewicz, Danczak and Ligocki2003; DuRant et al. Reference DuRant, Hopkins, Hepp and Walters2013); even a marginal increase over ambient temperature could result in shorter incubation periods. Shorter incubation periods mean that adult animals can be free of the nest sooner, whether they are incubating or guarding.
A key assumption of this experiment is that Troodon and its relatives were endothermic. Although a few modern ectotherms do contact incubate (Stahlschmidt and Denardo Reference Stahlschmidt and Denardo2009), it seems unlikely that Troodon shared these specialized behaviors. Histological evidence does support endothermy in Troodon (Varricchio Reference Varricchio1993). Additionally there is ample evidence to suggest that endothermy was widespread in dinosaurs, theropods specifically (Barrick and Showers Reference Barrick and Showers1994; Fricke and Rogers Reference Fricke and Rogers2000; Amiot et al. Reference Amiot, Lecuyer, Buffetaut, Escarguel, Fluteau and Martineau2006; Eagle et al. Reference Eagle, Tutken, Martin, Tripati, Fricke, Connely, Cifelli and Eiler2011). Recent research on troodontid body temperatures provides an estimated range of 28°C–38°C (Dawson et al. Reference Dawson, Field, Hull, Zelenitsky, Therrien and Affek2020). Ten degrees is a substantial difference, and a narrower band (35°C–40°C) has been proposed for the closely related oviraptorosaurs (Amiot et al. Reference Amiot, Wang, Wang, Lécuyer, Mazin, Mo, Flandrois, Fourel, Wang, Xu, Zhang, Zhou and Benson2017). These temperatures fall within the range of modern birds, including emus (Dromaius novaehollandiae) and ostriches (Struthio camelus), terrestrial birds of comparable sizes to troodontids and oviraptorosaurs (Szczerbińska et al. Reference Szczerbińska, Majewska, Tarasewicz, Danczak and Ligocki2003; Hassan et al. Reference Hassan, Siam, Mady and Cartwright2004).
Furthermore, despite the fact that no feather fossils can currently be directly assigned to T. formosus, it is assumed that it had feathers similar to those found in other troodontids, oviraptorosaurs, and indeed theropods in general (Hopp and Orsen Reference Hopp, Orsen, Currie, Koppelhus, Shugar and Wright2004; Barrett et al. Reference Barrett, Evans and Campione2015). This assumption is important in that feathers would have provided insulative benefits for the nest microenvironment. This experiment does not attempt to investigate endothermy or the presence of feathers in Troodon. Instead, the purpose is to investigate the efficiency of contact incubating partially buried eggs under the assumptions that adults exhibited some form of endothermy and insulation.
Nomenclatural Note
There has been some discussion on the validity of the name Troodon formosus. Most recently, van der Reest and Currie (Reference van der Reest and Currie2017) have suggested abandoning the name, reverting to Stenonychosaurus inequalis (a junior synonym), and designating a neotype. The name T. formosus is still used herein, as only the International Commission on Zoological Nomenclature can make a neotype ruling while an original holotype exists (ICZN 1999: art. 75). Furthermore, the continued use of the T. formosus supports stability—the underlying goal of the ICZN.
Methods
To investigate this brooding conundrum, an artificial system was constructed to replicate a nesting microenvironment similar to what might have been present for Troodon. A sediment-filled box was used during the indoor trials to represent a nesting location. Figure 1A shows the box, with eggs in the middle. These were nonviable chicken eggs with inserted thermometers. Additional thermometers were placed at various depths in the sediment, outside the experimental box for ambient temperatures, and within the air gap of the microenvironment. In this experiment, the air gap is the space between the two eggs, under the surrogate, and above the sediment. A surrogate dinosaur was created using a vinyl-held water bath warmed by an aquarium heater. This surrogate had a layer of insulation around the outside, top, and edges of the bottom. After controlled indoor trials, the same surrogate was used to conduct a similar test outside. In a natural setting the ground is a practically infinite heat sink—its overall temperature will not change no matter how long an incubating parent rests there. The local temperature does change though, and energy will continually flow from the adult into the surrounding nest and from the nest into deeper earth.

Figure 1. Photo series showing the surrogate dinosaur incubator, indoor sediment setup, and outdoor setup. The sediment container was 43 × 50 × 80 cm. The surrogate measured approximately 53 × 41 × 41 cm. A, A bird's-eye view of the sediment container, eggs, surface probes, and thermometer displays. In the final runs, the thermometer displays were localized and adhered to the side of the sediment container for ease of data capture. B, The surrogate—an insulated soft vinyl water container with water heater—on top of the sediment container. C, The eggs can be seen below the surrogate through the water held within the vinyl when the lid, insulation layer, and aquarium heater are removed (bird's-eye view). Tape covers the top to keep the thermometers in place within the eggs. D, An image of the surrogate outside in the testing area near Bozeman, Montana, at an elevation around 1850 m.
The indoor experimental system was of a size and situation such that energy could continually flow from the surrogate into the environment without raising ambient temperature. If too small, the environment will slowly heat up and provide skewed results. This state can be assured if the periphery sediment maintains a similar temperature to that of the ambient throughout the experiment. If this is the case, then the total effective heating zone of the surrogate dinosaur can be investigated.
A 43 × 50 × 80 cm plastic container was filled with approximately 272 kg of multipurpose sand (Sakcrete brand). Grains were well mixed and ranged from clay to coarse sand, with a small amount of gravel as well. Paleosols from the Two Medicine Formation were thought to be representative of well-drained soils (Retallack and Wolberg Reference Retallack, Wolberg, Wolberg, Stump and Rosenberg1997), and a lack of soil moisture is further evidenced by the abundance of fossil pupa cases found in that horizon (Martin and Varricchio Reference Martin and Varricchio2011; Freimuth and Varricchio Reference Freimuth and Varricchio2019). Accordingly, the sediment for this experiment was kept dry. The sediment container rested on a solid cement floor in a temperature-controlled room. No basement, floors, or utilities were located below the experimental area. Room temperature was set to 15.5°C and monitored independently of the thermostat.
A representative surrogate dinosaur was imagined as a heated water bath (Fig. 2). This bath was constructed out of PVC framing that held a vinyl-lined interior filled with approximately 19 kg of water. Vinyl was chosen as a soft water-holding material that could contact the eggs without undue pressure—similar to skin. The water bath itself was wrapped with approximately 3 cm of home insulation on the sides, top, and bottom edges. The egg contact face was left as just vinyl. Importantly, no insulation was used in the box that held sediment. The outside of the surrogate was wrapped in soft liner to help hold the insulation together. Final dimensions of the surrogate were approximately 53 × 41 × 41 cm. An aquarium heater (Hygger 200 watt submersible heater) heated the water bath to 36.8°C (which equalized to 34.4°C) for the indoor trials and 35°C (which equalized to approximately 29°C) for the outdoor.
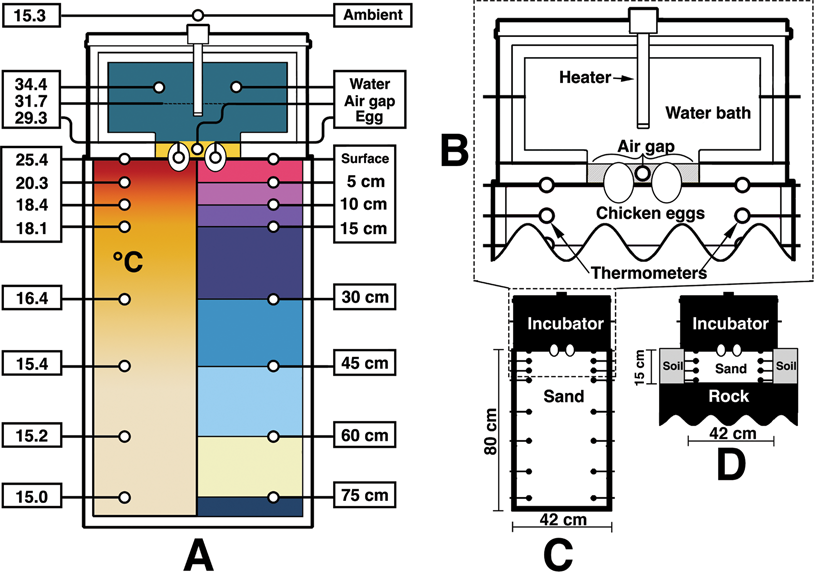
Figure 2. Diagram of the surrogate incubator and sediment container. A, A cross section of the experiment with temperatures (°C) corresponding to those recorded at 72 hours (left). The right side of A shows the location of temperature-probe groups. Divisions correspond to those used in Figs. 3–5. B, A simplified pullout of the surrogate incubator, eggs, and sediment interface. C, Dimensions of the sediment container. D, The surrogate over the sand-filled chamber in the outside setup.
Temperature probes (Aquaneat aquarium digital thermometers) were tested beforehand for regularity among each other and in different substrates, as they themselves could not be independently calibrated. All thermometers measured within 0.3°C from the median during the indoor trials and 0.5°C for the outdoor. For the ambient temperature controlled indoor experiment, a total of 25 thermometers were used. Two were placed at each measurable location: the water bath of the surrogate, air ambient, eggs (one thermometer in each), interior nest air gap, surface, and then within the sediments at depths of 5 cm, 10 cm, 15 cm, 30 cm, 45 cm, 60 cm, and 75 cm. Locations are shown in Figure 2A along with final temperatures recorded at those locations after 72 hours. Temperatures were recorded for 72 hours for the indoor trials and 48 hours for the outdoor. Energy input was recorded via a plug-in kilowatt-hour electricity monitor (Poniie PN2000) that all electrical energy flowing into the system passed through.
The same surrogate dinosaur was used for the outdoor trials. A hole was dug outside with a length and width slightly greater than that of the surrogate dinosaur. It was dug to a depth of 15 cm and filled with the same sand. The hole only extended slightly 15 cm due to the shallow soil profile and close bedrock. Thus, the bottom of the sand-filled hole was partially in contact with the rock. This created a local nesting environment likely less favorable than a troodontid would have used, as nest energy would leach faster through solid rock than air-pocketed sand and sediment. Regardless, 15 cm was the experimental zone to be investigated, as the majority of thermal fluctuation during the indoor experiments fell within this distance. Once the hole was dug and filled, thermometers were placed to measure temperatures at depths of 5 cm, 10 cm, and 15 cm. Additional thermometers were positioned to measure the surrogate, air ambient, eggs, interior nest air gap, and the surface sediment.
The eggs selected for this experiment were store-bought chicken eggs, each with a mass of 56 g. These eggs were vertically oriented and buried halfway in the sediment. For this study the embryos were nonviable, as only temperature was being investigated. Developmental rate of embryos is directly related to temperature, so examination of temperature potentially provides information on those rates (Szczerbińska et al. Reference Szczerbińska, Majewska, Tarasewicz, Danczak and Ligocki2003). Holes were tapped into the tops of the eggs to allow thermometers to be placed inside, and then the holes were sealed over.
To get an idea of possible egg temperatures from higher-temperature incubation situations, the average ambient temperature was compared with the average surrogate temperature over the final 16 hours of each trial. The difference between the surrogate maximum and ambient minimum was then calculated and compared with difference between the average egg temperatures and ambient temperatures for those time periods. These two differences were then compared to arrive at a useful percentage value. This value takes the disparity between the ambient and body-surrogate temperature into account, providing a more useful contrast than the arbitrary designation of 0°C as the temperature floor (and any comparisons using kelvin are compressed and misleadingly similar due to the additional 273 degrees). With these values derived, hypothetical temperature ranges for different ambient environments were calculated alongside 35°C and 40°C incubators (Table 1).
Table 1. Derived approximate egg temperatures for different ambient–body temperature combinations. Actual experimental body, ambient, and egg temperatures are italicized. 10.0 = Cretaceous Arctic cool, temperate coldest month, outdoor experimental; 15.7 = Cretaceous Arctic moderate, indoor experimental; 22.0 = Cretaceous Arctic warm, temperate moderate; 27.0 = temperate warm; 29.0 = temperate hot.

The slopes of the best-fit lines were compared to ascertain the similarity of the rates of change between the indoor and outdoor trials. If the rates are equal, then the slopes would be identical and the lines parallel. A normalized dot product calculation was used to test how close to parallel each line was. The dot product (U⋅V) of the two vectors (U and V) can be found with:
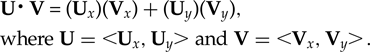 $${\eqalign{&{ \rm \bf U\middot V} \equals \lpar {\rm \bf U}_x\rpar \lpar {\rm \bf V}_x \rpar + \lpar {\rm \bf U}_y \rpar \lpar {\rm \bf V}_ y \rpar, \cr&{\rm where} \;{\rm \bf U} = \;\lt \!\! {\rm \bf U}_x, {\rm \bf U}_y \!\! \gt {\rm and} \;{\rm \bf V} =\; \lt \!\! {\rm \bf V}_x, {\rm \bf V}_y \!\! \gt \! .}$$
$${\eqalign{&{ \rm \bf U\middot V} \equals \lpar {\rm \bf U}_x\rpar \lpar {\rm \bf V}_x \rpar + \lpar {\rm \bf U}_y \rpar \lpar {\rm \bf V}_ y \rpar, \cr&{\rm where} \;{\rm \bf U} = \;\lt \!\! {\rm \bf U}_x, {\rm \bf U}_y \!\! \gt {\rm and} \;{\rm \bf V} =\; \lt \!\! {\rm \bf V}_x, {\rm \bf V}_y \!\! \gt \! .}$$If the vectors are first normalized, then a resulting dot product of 1 means the lines are exactly parallel, whereas a dot product of 0 means they are orthogonal. Vectors were taken from the trend lines of the rising egg temperatures from the first hour of the indoor (y i = 0.573x + 17) and outdoor (y o = 0.817x + 11.9) trials. This was repeated for the trend lines from hours 8 to 48 (y i = 0.184x + 28.3 and y o = 0.319x +24.3).
These experiments were conducted outside the city of Bozeman, Montana, at an elevation of approximately 1850 m. The outdoor trials occurred during the week of October 6, 2019. Temperature readouts were recorded, tabled, and graphed within Microsoft Excel. Figures were compiled in Adobe Photoshop.
Results
Indoor Trials
During indoor tests the water bath temperature dropped from 36.8°C (Fig. 3). After 1 hour it was down to 33.7°C, and it stayed within ±0.7°C until the end of the 72 hour run. The ambient temperature fluctuated between 17°C and 14.9°C with a median of 15.7°C. Egg temperature began at 16.2°C and rose to 23.4°C within one hour. It continued to rise up to 29.3°C by 56 hours and stayed there until 72 hours. Average egg temperature was closer to that of the water bath/surrogate than ambient by 2 hours into the trial and remained there until the end.

Figure 3. Combination area and line graphs showing energy throughout the temperature-controlled indoor experiments. Values averaged between probes at the same locations. An area chart is used to show the difference in temperatures between two adjacent zones. Actual temperature values are tracked by the upper border of a color (water/surrogate and body temperature being the same—body temperature is emphasized for comparison to egg and ambient). A, Readings during the first 60 minutes of testing. It is difficult to visually differentiate depths below 15 cm due to the closeness of temperatures. B, Rising temperatures over hours 2 through 8. C, Data begin at 8 hours and ends at 72 hours. The y-axis is the same throughout. D, Pullout highlighting the difference in energy within the first 15 cm of sediment vs. the remaining 60 cm. After 2 hours of incubation, egg temperatures remain closer to the water/surrogate than ambient temperatures. Note that the y-axis base is truncated to better show changing temperatures.
The temperature of the air gap between the surrogate and the sediment surface was the only area warmer than the eggs after 20 minutes of testing. By 24 hours, this temperature was 31.6°C, and it stayed within 0.1°C until the end of the trial. Within the sediment, the majority of the temperature changes occurred within the first 15 cm. After 72 hours, there was a 7.3°C difference between the surface of the sediment and 15 cm, while there was only a 3.1°C difference between 15 cm and 75 cm (more than half of which was between 15 and 30 cm). Temperatures at 60 cm fell between 15.1°C and 16.0°C with a median of 15.5°C. Values at 75 cm were similar, ranging between 14.7°C and 15.7°C with a median of 15.2°C. During the course of the experiment, the water heater warming the surrogate used on average 22.7 kcal/h.
After the completion of the 72 hour run, the surrogate was removed from the sediment container, and cool-down temperatures were recorded for 8 hours (Fig. 4). Surface temperature fell rapidly over the first 30 minutes—from 25.4°C to 20.4°C. All sediment temperatures below 5 cm changed 0.1°C or less over the first hour. Egg temperatures dropped from 29.3°C to 25.5°C in the first half hour, and then down to 22.75°C after an hour. Within 5 hours, all temperatures were within approximately 2.5°C of ambient values.
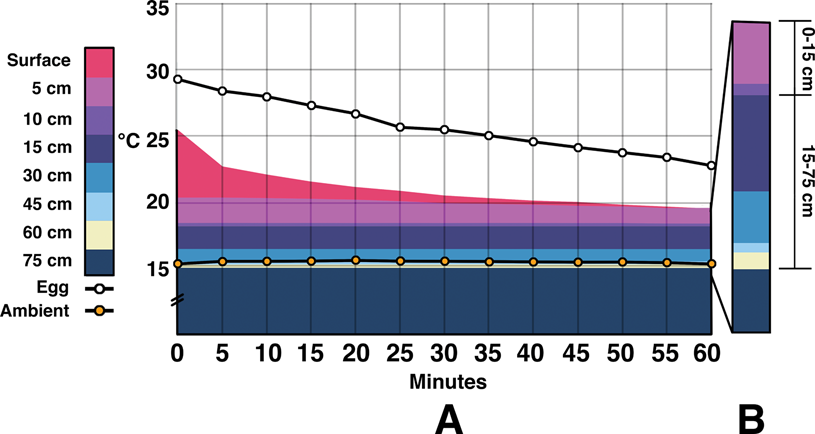
Figure 4. Combination area and line graph showing temperatures once the surrogate had been removed from the sediment container during the indoor trials, hence the omission of water/surrogate temperature. A, Decreasing temperatures over a 60 minute period. Within the first 5 minutes, there is a rapid decline in the temperatures of both the nest air gap and surface sediments. The eggs show a fairly constant decline in temperature throughout the hour. Temperatures at depths below 5 cm barely fluctuate during this period. B, Pullout showing energy differences within the first 15 cm vs. the remaining 60 cm (contrast with Fig. 3D).
Outdoor Trials
Water temperature during the outdoor portion of this experiment began at 35°C and stabilized around 29°C by 3 hours (Fig. 5). It then fluctuated ±0.7°C for the remainder of the experiment. Ambient temperature was highly variable throughout the day, with a recorded high of 22.5°C and a low of 2.1°C. The median was 9.8°C. Egg temperature rose steadily, beginning at 10.7°C and reaching 20.7°C by the first hour. The eggs fluctuated between 24°C and 26.5°C after the fifth hour and continued to do so throughout the remainder of the trial.
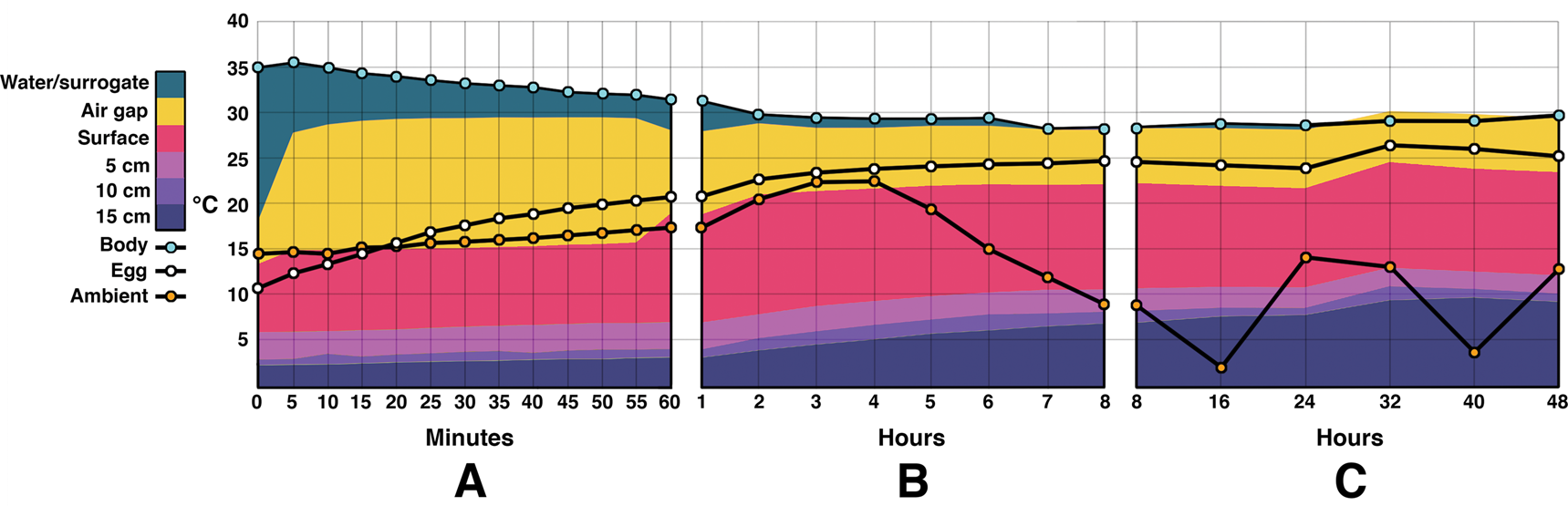
Figure 5. Combination area and line graphs showing energy flow during the outdoor experiments. Time zero was noon. Temperatures at depths below 15 cm were not measured for this experiment. A, Temperatures during the first 60 minutes. Water/surrogate temperature decreases steadily while egg temperatures rise. Sediment temperatures rise slowly. B, Temperature changes from the 1 hour to 8 hour mark. The water/surrogate temperature mostly levels off after a few hours. Sediment and egg temperatures continue to rise. Ambient temperature rises until 4 p.m. and then begins decreasing. C, The remaining 40 hours after the initial 8 hour test set. The nest air gap temperature briefly overtakes the water temperature, possibly due to a discrepancy in the insulation of the water bath vs. the egg chamber. Ambient temperature fluctuates daily, while the water/surrogate temperature remains fairly constant. Egg temperatures remain close to water/surrogate despite fluctuating ambient temperatures. Note that the y-axis is not truncated as in Fig. 3.
The temperature of the air gap rose faster and stayed higher than those of the eggs. It jumped from 18.1°C to 28.6°C within the first 10 minutes, and then stayed within 1.5°C for the rest of the experiment. The temperatures recorded at the 15 cm depth did rise continuously throughout the experiment, from 2.3°C to 9.65°C at 40 hours, falling back about 0.5°C by 48 hours. During the outdoor trials the water heater used an average of 18.27 kcal/h.
Table 1 was created to show possible egg temperatures for ambient–body temperature combinations beyond the scope of this study. Values were calculated by comparing the difference between ambient and egg temperatures with the difference between ambient and body temperatures (see “Methods”). The following values were derived for warmer incubators and differing ambient environments. An incubating adult with a body temperature of 35°C would possibly see egg temperatures of 29.9°C to 33.4°C, while an incubator with a body temperature of 40°C would have egg temperature ranges of 33.9°C to 37.4°C (Table 1).
Discussion
Actualistic investigations will always include assumptions and generalizations, and their results should be interpreted carefully. For these experiments it was assumed that an incubating Troodon-like animal would be endothermic with an insulating integumental covering. Because the purpose of this study was to test the thermal properties of partially buried eggs, a nest structure was not replicated. Trace fossils linked to T. formosus show nests with a circular raised rim (Varricchio et al. Reference Varricchio, Jackson, Borkowski and Horner1997). While nest structure certainly affects egg microclimate, it was outside the scope of this study. Regarding insulation, final temperatures from the trials showed that eggs maintained a temperature difference from the ambient that was 75.6% of what the surrogate maintained for the indoor trials and 83.1% for the outdoor. These percentages were calculated by finding the difference between the egg and ambient temperatures, then dividing it by the difference between the surrogate and ambient temperatures (same technique used for Table 1). Temperature recordings within active eider (Somateria mollissima) and barnacle goose (Branta leucopsis) nests (Rahn et al. Reference Rahn, Krog and Mehlum1983) found that the eggs hovered around 84.3% and 86.8%, respectively, when compared with ambient and body temperatures in the same manner. Although this may be coincidental due to the large difference in parental mass and nest structure, the similar ratios suggest that the surrogate dinosaur is not unrealistically efficient.
Egg temperatures did equalize more closely to surrogate values than ambient, but the final egg temperatures (averaged over the final 16 hours of each experiment) for the indoor (29.3°C) and outdoor (25.9°C) tests are low for actual incubation. Though it is probable that dinosaurs would have had a lower incubated egg temperature than most modern birds, due to longer incubation times (Varricchio et al. Reference Varricchio, Kundrat and Hogan2018), the experimental values are still likely cooler than actual. These cooler incubation temperatures can be explained by the experimental parameters. First, one limitation of the experiment was the water heater used to warm the surrogate. Once placed on the sediment, the heater struggled to maintain temperatures of 35°C–40°C. Equalized surrogate temperatures for each of the trials (33.7°C indoor and 29°C outdoor) were several degrees lower than hypothesized body temperatures for oviraptorosaurs (Amiot et al. Reference Amiot, Wang, Wang, Lécuyer, Mazin, Mo, Flandrois, Fourel, Wang, Xu, Zhang, Zhou and Benson2017), but within the range suggested by Dawson et al. (Reference Dawson, Field, Hull, Zelenitsky, Therrien and Affek2020) for troodontids. Though the surrogate temperature was on the low side of the spread, the fact that the eggs still maintained temperatures significantly closer to the water bath than ambient provides strong support for the possibility of incubating partially buried eggs. Energy leaching from the eggs into the sediment did not outpace replenishment from the surrogate, even at these lower temperatures. Higher surrogate or body temperatures would likely raise the temperature at which the eggs would equalize. The discrepancy between set water temperature, starting water temperature, and equalized water temperature was difficult to bridge in this experiment. A more powerful water heater would likely be able to maintain higher and more consistent surrogate temperatures to improve future investigations.
The ambient temperatures used for each experiment were also likely lower than what an incubating T. formosus would have endured. The outdoor average ambient temperature was 9.8°C, with a low of 2.1°C. This falls within the range for the coldest month of a temperate climate (−3°C to 18°C with an average of 10.5°C) (Kottek et al. Reference Kottek, Grieser, Beck, Rudolf and Rubel2006), and evidence suggests T. formosus lived in a warm temperate climate (Dodson Reference Dodson1971). It is unlikely that this animal would have been incubating during the coldest month of the year. Even the indoor controlled ambient average of 15.7°C is likely lower than what T. formosus would have experienced during incubation season. Research by Burgener et al. (Reference Burgener, Hyland, Huntington, Kelson and Sewall2019) reconstructs a mean annual range in temperature of 21°C to 27°C for paleoclimates of the Two Medicine Formation, further suggesting that experimental ambient temperatures were probably lower than actual. Nevertheless, troodontid material has been found in Arctic paleolatitudes (Fiorillo et al. Reference Fiorillo, Tykoski, Currie, McCarthy and Flaig2009) with warm weather temperatures of 19°C (Golovneva Reference Golovneva2000). So, while 15.7°C might be a reasonable temperature for Arctic incubation, it is likely lower than what more southern troodontids would have experienced in the Cretaceous.
Despite these colder values, the eggs did stay closer to surrogate than ambient temperature after the initial heating phase. The loss of energy to the sediment did not outpace replenishment from the surrogate in either the controlled or outdoor environment. Even with unfavorably cool conditions, contact incubation appears to have conferred a thermodynamic advantage to partially buried eggs. Although Troodon nests lacked the material complexity of modern bird nests, it seems that even a simple air gap between parent and substrate could have created a beneficial microclimate. Perhaps nest insulation becomes more important as the mass of the incubating parent decreases. While this study is certainly insufficient to determine whether or not small theropod dinosaurs incubated their eggs, it does seem to support their capacity to do so even in the partially buried nest structures that have been identified.
The slope of the best-fit lines for egg temperatures, indoors and outdoors, were similar when measured during the first hour and from hours 8 to 48. The normalized dot product of the indoor and outdoor vectors over the first hour was 0.987, and it was 0.992 for the latter 40 hour segment. This suggests comparable rates of change whether indoors or outdoors (if normalized dot product = 1, then the lines are exactly parallel). It appears that overall the eggs increased toward surrogate temperature largely independent of outdoor variation. During the 48 hour outdoor trials, there appear to be slight increases or decreases in temperature that could perhaps correspond to the ambient increase or decrease in the previous 8 hours (figure 4). Despite this lag, the eggs remained closer to surrogate than outdoor ambient temperatures. It seems that the indoor experiment should be an acceptable testing analogue given the similar overall rates of change.
It is important to note that while partially buried eggs received a significant temperature boost from the surrogate, depths below 15 cm showed little change in energy. Below this thermal-input threshold there is certainly a point at which buried eggs would not receive any heat-related benefit from an incubating adult. Though the top 15 cm was critical in this experiment, that thermal input threshold is likely dependent upon the mass of the incubating parent, climate, and nest structure. A parent incubating eggs buried in layers at different depths in the sediment would see eggs at varied temperatures. Presumably this would lead to asynchronous hatching if the temperature banding and egg layers were distinct enough.
Relatedly, recent research by Yang et al. (Reference Yang, Wiemann, Xu, Cheng, Wu and Sander2019b) suggests that oviraptorosaur nests may have been incompatible with contact incubation. The authors reconstruct a volcano-shaped multitiered nest that they argue was likely too large for the adult to effectively cover with its body. Due to the stacked arrangement of eggs, the authors also thought it unlikely that a parent could effectively contact each egg in the clutch. Eggs were layered within the nest in distinct rings, with their blunt ends exposed to the inner air gap of the structure. Other researchers suggest that oviraptorosaurs did contact incubate, or at least brood (Norell et al. Reference Norell, Balanoff, Barta and Erickson2018). Their newly described specimen, consisting of an adult-associated Citipati osmolskae clutch, shows the same brooding posture described in other nesting oviraptorosaurs. Results obtained from this T. formosus nest study suggest that if an adult oviraptorosaur could comfortably cover the nest, there might still be a noticeable temperature gradient due to the depth of the nest, the adult heat source being at the top, and hot air's propensity to rise.
Due to this gradient, oviraptorosaur eggs at the bottom of a clutch may have experienced different temperatures than those at the top. Distinct temperature zones could have possibly affected hatching synchrony. If the eggs all began incubation at the same time, the uppermost eggs might have developed more quickly. However, the lower eggs were almost certainly the first to be laid. These eggs may have hatched first if incubation began before the clutch was complete. Yang et al. (Reference Yang, Engler, Lallensack, Samathi, Makowska and Schillinger2019a) describe embryonic oviraptorosaur remains that seem to support hatching asynchrony through more-developed bottom eggs. This does beg the question of the architectural stability of a stacked egg nest in which the bottom layer hatches first. It is also important to note that hatching synchrony could have varied significantly among species of oviraptorosaurs. Yang et al. (Reference Yang, Wiemann, Xu, Cheng, Wu and Sander2019b) note that their model does not readily align with any modern-day nests. It is even possible that a temperature gradient could have reduced hatching time discrepancy too, as the bottom eggs (though laid first) might have experienced cooler temperatures than their later-laid but warmer upper-level siblings.
Aside from keeping eggs closer to surrogate than ambient temperatures, another measure of efficiency for this study was the amount of energy used during the trials. If excessive electrical energy had been needed to warm the eggs, that would raise concerns about the calorie requirements for an adult dinosaur incubating in this manner. The indoor trials averaged 545 kcal/day while outdoors averaged 438 kcal/day. Surprisingly, fewer calories were used outside than inside, perhaps because the surrogate sealed better with the ground outside, being slightly wider than the sediment box used indoors. These values do not seem to be prohibitively high considering that emus have been measured to expend between 645 and 813 kcal/day while incubating (Buttemer and Dawson Reference Buttemer and Dawson1989).
Evidence indicates that the incubation periods in T. formosus would have been around 74 days (Varricchio et al. Reference Varricchio, Kundrat and Hogan2018) compared with only 56 days for emus (Buttemer and Dawson Reference Buttemer and Dawson1989). Total calories expended by the incubating emu during the period of attendance, using averaged values, yields 40,824 kcal. This experiment, if run for 74 days using the higher-end caloric requirements, gives 40,330 kcal. The convergence here, of course, does not suggest that T. formosus and emus might expend the same amount of energy while incubating, but rather that the energy used for the experiment does not appear to be exceedingly high. If an incubating T. formosus did use a similar amount of daily energy (average 729 kcal) as an incubating emu, then its total caloric expenditure for the incubation period would be around 53,946 kcal. Still, proportionally, a 50 kg T. formosus spending 53,946 kcal is similar to a 41.45 kg emu (averaged mass of incubating emus from Buttemer and Dawson [1989]) spending 40,824 kcal: 1079 kcal/kg versus 985 kcal/kg. These comparisons would certainly benefit from more rigorous testing, but the longer incubation period suggested for T. formosus does not seem to require excessive caloric expenditure.
It has been suggested that perhaps theropod dinosaurs, even smaller ones, could have been too heavy to safely provide contact incubation for their eggs (Deeming and Mayr Reference Deeming and Mayr2018). In this study the chicken eggs were able to safely sit under the 19 kg water bath, an amount above their calculated load mass (Ar et al. Reference Ar, Rahn and Paganelli1979). Of course, a substantial portion of the incubator's weight rested on the ground, but perhaps a similar strategy could have been employed by brooding dinosaurs. Certain postures would allow the fraction of an adult's mass not supported by limbs and ground to be distributed among all eggs in their clutch, substantially reducing pressure applied to any individual egg. As such, a comparison of egg-load mass and adult mass could possibly be improved by dividing the adult mass by probable number of adult-contacted eggs in the clutch. This consideration takes distributed force and resulting pressure into account, the same way that a single nail can easily penetrate skin, but you can lie on hundreds of nails without any punctures—such as with the classic bed of nails demonstration. Research by Zhao and Ma (Reference Zhao and Ma1997) shows that eggs from troodontids were oriented in such a way as to maximize their load-bearing strength. Additionally, it is possible that adults directed a sizable portion of their weight to the ground via contact between the ground surface and two or four limbs. Such a resting posture could be similar to that shown in theropod trace fossils (Milner et al. Reference Milner, Harris, Lockley, Kirkland and Matthews2009).
Recent research shows that eggs belonging to the ootaxa Spheroolithidae, Megaloolithidae, and Faveoloolithidae were frequently associated with specific sediment profiles (Tanaka et al. Reference Tanaka, Zelenitsky, Therrien and Kobayashi2018). On the other hand, troodontid eggs (Prismatoolithidae) were not affiliated with any particular lithology. This lack of preference was also true for oviraptorosaurs, enantiornithines, and neornithes. The authors suggest that this discrepancy corresponds to incubation modes, that burial and mound nesters were discerning with nesting sediments, while contact incubators were not. This could suggest that perfectly matching sediments when modeling contact incubation behavior may be unnecessary.
Troodontid fossil material has been found at 76.7°N, latitudes well within the Arctic Circle (Fiorillo et al. Reference Fiorillo, Tykoski, Currie, McCarthy and Flaig2009). Tanaka et al. (Reference Tanaka, Zelenitsky, Therrien and Kobayashi2018) also investigated likely nesting habits at these polar paleolatitudes. The authors suggest that dinosaur fossils from these latitudes correspond to species exhibiting mound nesting or partial burial. Paleoclimate data from similar paleolatitudes suggest a cold weather average of 3°C, warm weather average of 19°C, and an annual average of 10°C (Golovneva Reference Golovneva2000). It seems unlikely that open-faced nests could have worked at these temperatures without contact incubation to warm them. While mound nesting is a possibility in these environments (Tanaka et al. Reference Tanaka, Zelenitsky, Therrien and Kobayashi2018), no evidence supporting mound nesting has yet been found for troodontids (Varricchio et al. Reference Varricchio, Jackson and Trueman1999).
Path from Ectothermic Guarding to Contact Incubation
While it is still debated whether contact incubating behavior and partially buried eggs overlapped, this study indicates that it is possible that contact incubation of partially buried eggs conferred a benefit. This potentially opens up a simple model for the evolution of the subaerial incubation condition that modern birds have elaborated on to great effect. First, the ancestral condition is assumed to be the guarding of subterranean eggs. Plenty of modern animals guard buried eggs, including crocodilians and megapodes (Jones et al. Reference Jones, Dekker and Roselaar1995; Thorbjarnarson Reference Thorbjarnarson1996). Buried nests also appear to be relatively common in dinosaurs (Deeming Reference Deeming2006). Hypothetically, there are two behavioral paths that would take an organism from guarding subterranean eggs to contact incubating subaerial eggs. One path would require subaerial egg behavior to evolve before contact incubation. The combination of simple guarding and subaerial eggs seems problematic, as the organism would trade off subterranean protection from predators and temperature fluctuation without gaining any obvious advantages. As merely a guard, the organism itself would not serve to ameliorate any of the temperature-related benefits lost from a subterranean nest.
The second path would have incubation behavior evolve before subaerial eggs. In this scenario, a guarding endothermic adult might slightly warm buried eggs just by spending time in the location of the nest. While certainly not as efficient as the contact incubation of subaerial eggs, even a small amount of energy input into a subterranean nest could help to further increase egg temperatures. Because temperature is often the determining factor of incubation rate and hatchling success (Tombre and Erikstad Reference Tombre and Erikstad1996; Martin et al. Reference Martin, Auer, Bassar, Niklison and Lloyd2007; DuRant et al. Reference DuRant, Hopkins, Hepp and Walters2013), a small increase in temperature could confer significant enough advantages over the ancestral state. Figure 6 shows how, given an endothermic adult and natural variation in egg burial depth, modern contact incubation behavior could gradually evolve. Once the trend begins, it is easy to see how indirect incubation could lead to weak and finally strong contact incubation behaviors. Eggs buried closer to the surface would gain increasing temperature benefits without sacrificing parental protection. Contact incubation does require significant sacrifices from the parent though, as it becomes more vulnerable to predation and less able to forage for food. It is not suggested that this entire transition occurred only once or solely within nonavian dinosaurs, as buried (Kurochkin et al. Reference Kurochkin, Chatterjee and Mikhailov2013) and partially buried (Fernandez et al. Reference Fernandez, Garcia, Fiorelli, Scolaro, Salvador, Cotaro, Kaiser and Dyke2013) eggs are found in fossil birds.
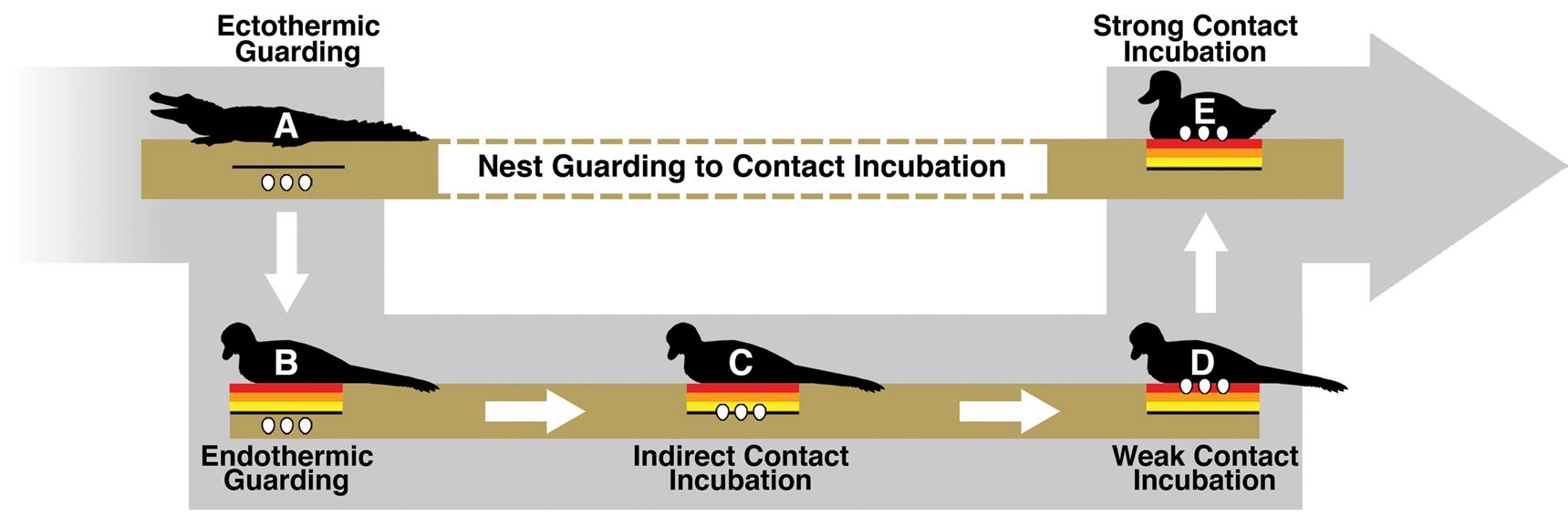
Figure 6. Illustration of a potential avenue for the evolution of modern strong contact incubation behavior. A, An ectotherm guarding its egg clutch, a frequent habit of both fossil and modern reptiles. B, An endothermic animal guarding its buried clutch and warming the ground below. In B, the eggs would not gain any temperature-related benefit from the adult. However, due to natural variation in burial depth, some nests within a population might eventually be buried close enough to the heating zone that they experience a small temperature increase from the adult body heat, as shown in C. This could be considered indirect contact incubation, where an adult primarily functions as a guard but a small amount of energy is incidentally reaching the buried clutch. Eggs experiencing a slight increase in temperature could feasibly hatch earlier—leaving the clutch and adult vulnerable for a shorter period of time. It is perhaps possible that an adult in this model could provide some insulative benefits from extreme weather conditions, but more likely the ground is doing the bulk of the thermoregulatory work. Eventually indirect contact incubation could lead to weak contact incubation, such as in D. Partially buried eggs within a troodontid nest would fall into this weak contact incubation category, where eggs gain a significant amount of energy from the adult but are not yet in a fully subaerial position. E, Strong contact incubation, such as in most modern birds, where eggs can experience maximum energy input and temperature regulation from a contact-incubating adult.
Conclusion
Birds could not have evolved such complex and varied nests without first breaking free from the subterranean tendencies of their reptile ancestors. Our understanding of dinosaur nesting habits is complicated by their position between these two very different groups. The blueprint for subaerial nesting behavior had to begin somewhere, and it is possible that dinosaurs were the pioneers. The partially buried eggs of T. formosus might be evidence of intermediate behavior, yet the ability of T. formosus to contact incubate in such a system is controversial. Although this simple study does little to confirm whether or not contact incubation behavior coincided with partly exposed eggs in theropods, it nevertheless sheds light on dinosaur nesting possibilities. These experiments suggest that the presumed inefficiency of contact incubating partially buried eggs may be misguided, as even half-buried eggs maintained temperatures closer to the surrogate than ambient in multiday trials under conditions likely cooler than those many troodontids would have experienced in the Cretaceous. Additionally, metered electrical input showed that the energy needed to keep the experimental eggs above ambient temperatures was not prohibitively high, as compared with modern emus. Nevertheless, there was a distinct temperature profile within the sediment. A thermal input threshold would likely appear in any nest with partially or fully buried eggs, where eggs buried below that limit would receive little to no warmth from an incubating adult. Still, the fact that even completely buried eggs could benefit from an incubating parent at certain depths implies a possible path for the evolution of subaerial nesting behaviors from more ancestral subterranean ones. Such intermediate nests might not have been as warm or efficient as those of modern birds, but they may have been sufficient to push the behavior forward.
Acknowledgments
J.H. would like to thank J. M. Hogan and K. Klayton for facility space to run the experiments unhindered, L. B. Huizenga for her relentless support, T. Lucille for assisting in the design and construction of the experimental system, T. Hogan and N. Lee for their thoughtful review of an early draft, S. Wuu for her thorough text edits, and finally E. Behr for his patience and positivity.










