Introduction
In 1868 the botanist, physician, abolitionist and General-Consul of Zanzibar Sir John Kirk spoke of a rare monkey occurring in the island's forests (Forbes, Reference Forbes1894). The species was named that year in his honour; Kirk's or Zanzibar red colobus Piliocolobus kirkii, yet 150 years later this endemic species remains poorly known, with no systematic assessment of its population and distribution having been attempted prior to this study. Nonetheless, P. kirkii is considered to be one of the rarest primates in Africa, and is categorized as Endangered on the IUCN Red List (Struhsaker & Siex, Reference Struhsaker and Siex2016).
The smallest of the three Piliocolobus species occurring in Tanzania (Davenport et al., Reference Davenport, Nowak and Perkin2014), and the one with the slowest reproductive rate (Siex & Struhsaker, Reference Siex and Struhsaker1999), the Zanzibar red colobus is a charismatic, group-living, arboreal monkey, restricted to Unguja, the principal island of the archipelago of Zanzibar (Struhsaker, Reference Struhsaker2010). Suggestions that the species occurred on mainland Tanzania (Rodgers, Reference Rodgers1981) have not been substantiated, although there is a small introduced population in Ngezi Forest Reserve on Pemba Island. It has been speculated that the monkeys were formerly distributed throughout Unguja, with higher densities in the west and central areas where deeper, more fertile soils supported higher forests, and lower densities in the coral thickets of the east coast (Pakenham, Reference Pakenham1984). A number of population estimates have been made (Table 1), from detailed density calculations in and around Jozani–Chwaka Bay National Park (Struhsaker & Siex, Reference Struhsaker and Siex1998a,Reference Struhsaker and Siexb; Siex & Struhsaker, Reference Siex and Struhsaker1999), in Kiwengwa Forest Reserve (Nowak & Lee, Reference Nowak and Lee2013; Johansen, Reference Johansen2016) and in Uzi/Vundwe (Nowak & Lee, Reference Nowak and Lee2013) and Masingini Forest Reserves (Khamis, Reference Khamis2010), to island-wide approximations of 1,469–2,400 individuals (μ = 1,774 ± SE 201).
Table 1 Total population estimates for the Zanzibar red colobus Piliocolobus kirkii on Unguja Island, Zanzibar (Fig. 1), and population/density estimates for individual sites.

* P. kirkii now extinct
With Unguja's forests disappearing at a rate of 19.4 km2 net loss per year (Kukkonen & Käyhkö, Reference Kukkonen and Käyhkö2014), ongoing increases in human population, tourism and residential development, and agricultural expansion, Zanzibar's biodiversity is threatened. To implement appropriate conservation measures for P. kirkii we needed to carry out an accurate and complete evaluation of the species’ distribution and abundance, and therefore we carried out systematic surveys to collect data for the first empirical assessment of the species’ conservation status. Forest primates are challenging to survey accurately, and various techniques have been suggested (Brockelman & Ali, Reference Brockelman, Ali, March and Mittermeier1987; Whitesides et al., Reference Whitesides, Oates, Green and Kluberdanz1988; Plumptre & Cox, Reference Plumptre and Cox2006; Rovero et al., Reference Rovero, Struhsaker, Marshall, Rinne, Pedersen and Butynski2006). However, based on our experience in Tanzanian forests with the kipunji Rungwecebus kipunji (Davenport et al., Reference Davenport, Stanley, Sargis, De Luca, Mpunga, Machaga and Olson2006) and the ashy red colobus P. tephrosceles we were able to adapt the methods we had designed and employed successfully (Davenport et al., Reference Davenport, Mpunga and Machaga2007, Reference Davenport, De Luca, Jones, Mpunga, Machaga, Kitegile and Picton Phillipps2008), to Zanzibar. These are the most precise and comprehensive methods to (a) determine the full distribution of P. kirkii, (b) census the total population of the species, and (c) provide the first comprehensive assessment of the species’ conservation status.
Study area
The study was carried out across the island of Unguja (1,529 km2) in the archipelago of Zanzibar in the United Republic of Tanzania. Much of Unguja is coral rag (Fig. 1), characterized by rocky outcrops and less fertile, shallow soils, providing little benefit to permanent agriculture (Hettige, Reference Hettige1990). This landscape is dominated by shifting cultivation and more natural vegetation. Protected areas and other sites surveyed are listed in Table 2 and included Jozani–Chwaka Bay National Park, Masingini and Kiwengwa Forest Reserves, Jambiani–Muyuni Proposed Forest Reserve, Kichwele, Dunga and Kibele Forest Reserve Plantations, as well as all other sites where P. kirkii has been recorded, suggested or was a possible inhabitant. Bungi Usalaama is an army barracks and was included as a proxy protected area for the purposes of this study, as no civilians are allowed within its boundaries. Habitats comprise mangrove, coral rag, thicket, groundwater forest, woodland and forest edge, plantations and gardens (shamba) at elevations between sea level and 110 m.
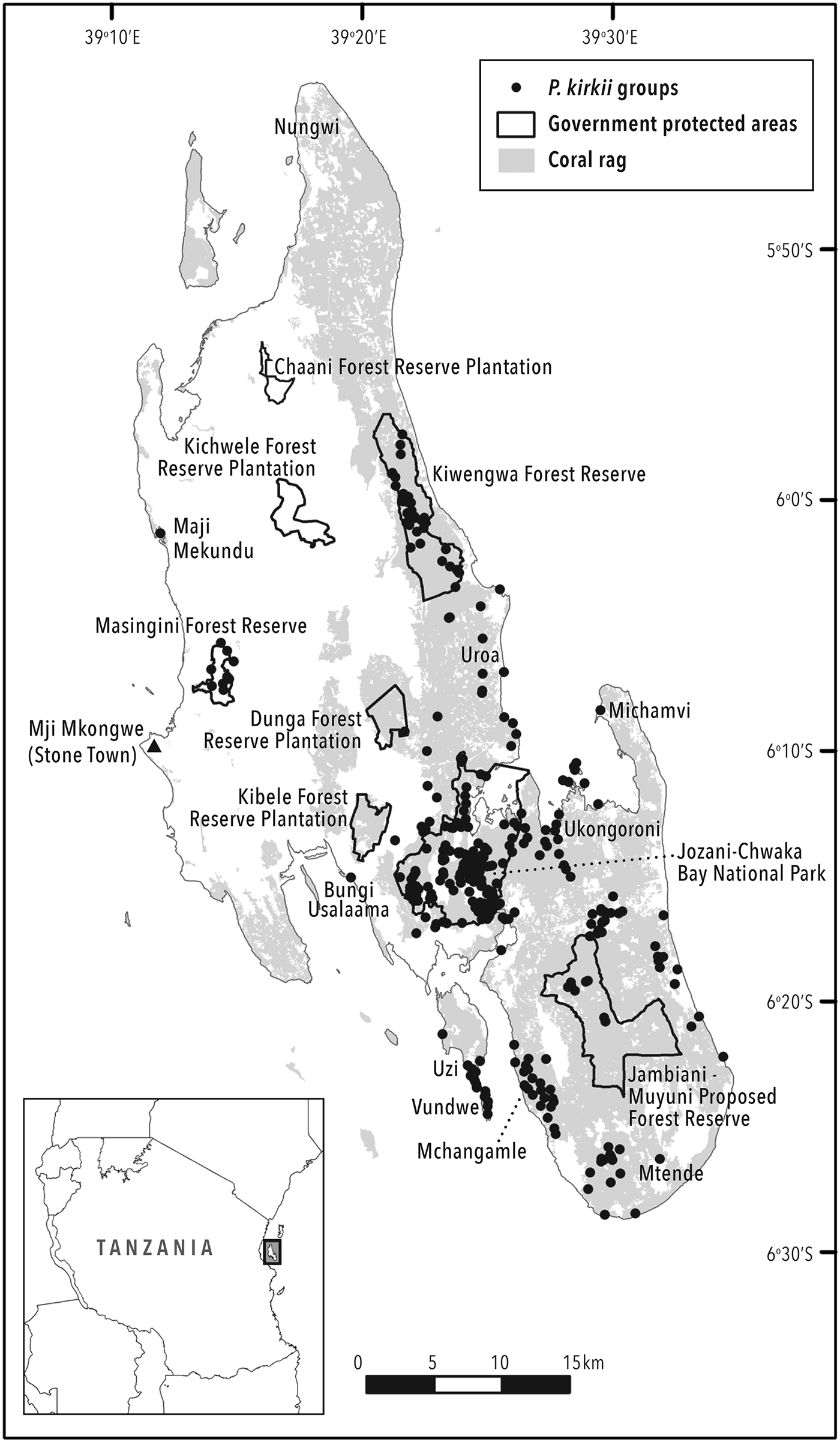
Fig. 1 Locations of all Piliocolobus kirkii groups located and counted on the island of Unguja, Zanzibar.
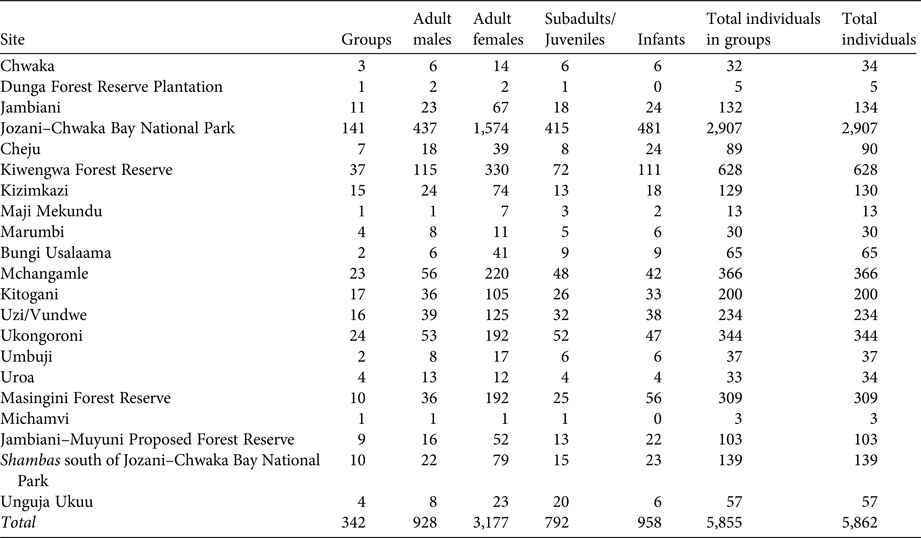
Table 2 Total number of P. kirkii groups, adult males, adult females, subadults/juveniles, infants, individuals in groups and total individuals per site, on Unguja Island, Zanzibar (Fig. 1).
Methods
During January 2013–January 2015 various methods were employed to determine distribution and abundance. Distribution data were collected using presence/absence surveys, whereas census data were recorded using total counts made while following groups.
Presence/absence surveys
Forests were selected for presence/absence surveys based on our prior knowledge of the areas, information from previous surveys, village interviews and the habitat type from which P. kirkii was already known. At each site, 3–4 pairs (teams) of observers searched concurrently for P. kirkii along separate pre-planned routes, using 1 : 50,000 topographic maps (Tanzania Surveys and Mapping Division, Series Y742), global positioning system (GPS) units and binoculars. Each team comprised a scientist and a field assistant. Only sightings were considered to be positive indicators of presence. New areas were surveyed each day, adjacent to the area covered the previous day. Some areas were revisited if poor weather hindered earlier work. Survey routes followed wildlife trails, human tracks and off-track, to survey a large area thoroughly. Each team walked slowly (c. 2 km per hour) and quietly during 06.30–18.00, no more than 100 m apart, scanning the understorey and canopy for monkeys. Surveys were paused in heavy rain. When an individual or group was detected, the observer remained until they were confident that the species was correctly identified as P. kirkii, and then the follow would begin.
Census
The methods employed were those we had devised previously and used successfully for complete census counts of the ashy red colobus in Sumbawanga (Davenport et al., Reference Davenport, Mpunga and Machaga2007) and the kipunji in the Southern Highlands (Davenport et al., Reference Davenport, De Luca, Jones, Mpunga, Machaga, Kitegile and Picton Phillipps2008). To ascertain the total P. kirkii population as accurately as possible, we adapted the complete count method, which is accepted as being the most precise primate census technique (Plumptre & Cox, Reference Plumptre and Cox2006; Davenport et al., Reference Davenport, Mpunga and Machaga2007, Reference Davenport, De Luca, Jones, Mpunga, Machaga, Kitegile and Picton Phillipps2008). Unlike gorilla census methods developed by Harcourt & Fossey (Reference Harcourt and Fossey1981) and McNeilage et al. (Reference McNeilage, Plumptre, Brock-Doyle and Vedder2001, Reference McNeilage, Robbins, Gray, Olupot, Babaasa and Bitariho2006), based on complete counts of indirect sign, our collection methods used direct observations of individuals only (Davenport et al., Reference Davenport, Mpunga and Machaga2007, Reference Davenport, De Luca, Jones, Mpunga, Machaga, Kitegile and Picton Phillipps2008). In this way our calculation of the population was neither an estimate nor an extrapolation based on density, but an absolute figure.
To count all individuals directly within every group, we aimed to locate and follow every group for a minimum of 3 consecutive days, tracking all movements and distances with a GPS. When a team located a group, it remained with the group at a distance that was sufficient to maintain contact while minimizing stress on the group (Cipolletta, Reference Cipolletta2003). Grid reference positions of the group were recorded routinely by GPS every 15 minutes. Teams maintained contact with one another via mobile phone. During the follows, the numbers of individuals, adult males, adult females, infants and subadults/juveniles in each group were counted eight times daily and/or whenever the opportunity arose.
The four field assistants had a combined total of 61 years’ experience (a mean of 15 years each) in P. kirkii research, including in determining sex and age-class. Extensive inter-observer reliability training was carried out in Masingini Forest Reserve prior to the study, and the same person did all the counting in each team, reducing potential errors resulting from a change of observers. Age classes were defined according to Siex (Reference Siex2003).
At any particular site, P. kirkii groups were considered to be unique if (1) they were seen at the same time by different observation teams, spending more than 75% of the observation time at a distance of at least 300 m apart (this was verified a posteriori); (2) one team saw a group other than the one they were following, at least 300 m away, and later verified that no other team had been near the group(s); (3) the groups were recorded > 300 m apart, at the same time, and subsequently moved in different directions. In cases where there was any doubt, at least two teams returned to the location at a later date to verify group identity through location, size and demography.
We used ArcGIS 9.3 (ESRI, Redlands, USA) to analyse observation data from all P. kirkii groups recorded in the census. Using these data we could calculate the area of occupancy (AOO), defined as the area within the species’ extent of occurrence (EOO) that a taxon occupies, excluding cases of vagrancy (IUCN, 2017). This definition reflects the fact that a taxon will not usually occur throughout its EOO, which may contain unsuitable or unoccupied habitats. We employed the grid method of AOO representation and calculation proscribed by IUCN (2017), whereby a 2 × 2 km grid of cells is overlain on observation points, although we acknowledge that with this grid size in Zanzibar, AOO may be overestimated. A taxon's EOO is the area contained within the shortest continuous boundary that can be drawn to encompass all the known, inferred or projected sites of present occurrence (IUCN, 2017). We measured the EOO by calculating the area of minimum convex polygons and using a geographical information system (GIS). As this measure excludes discontinuities within the total distribution, such as areas of unsuitable or heavily degraded habitat, we produced separate polygons for the isolated P. kirkii groups in Maji Mekundu, Masingini and Michamvi.
Results
The total effort spent searching for and following P. kirkii was 4,725 team hours. The surveys revealed the full extent of P. kirkii distribution across Unguja (Fig. 1). The introduced group in Ngezi on Pemba Island was not considered. Despite extensive surveys, P. kirkii was not recorded (and is presumed to be extirpated) from Bambi, Jendele and Kichwele Forest Reserves and Nungwi, where the species was once present. The species’ absence from these areas was supported by discussions in adjacent villages. One group only was found in each of Kibele and Dunga Forest Reserves and Maji Mekundu. We calculated an AOO of 376 km2 and an EOO of 428 km2. The census data and EOO yield a species-wide density estimate of 13.7 individuals per km2. P. kirkii distribution in terms of total time spent in each habitat type is illustrated in Fig. 2.
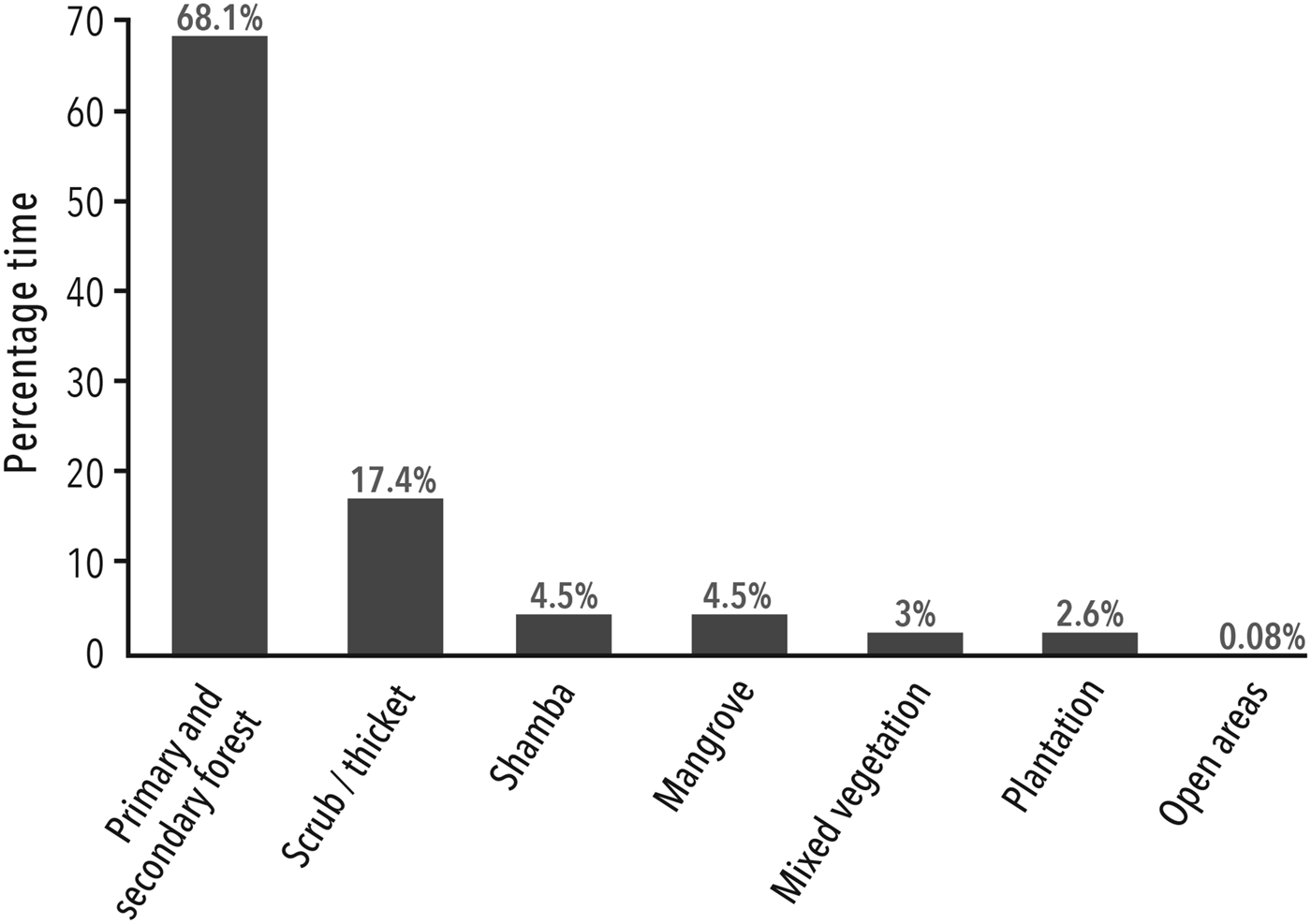
Fig. 2 Distribution of all P. kirkii groups according to time spent in each habitat type.
A total of 342 P. kirkii groups were identified during the census, as well as three singletons and four doubletons. The numbers of groups, individuals, adult females, adult males, subadults/juveniles and infants per site and site type are in Table 2. We estimate the total P. kirkii population to be 5,862 individuals, with 3–52 individuals per group (μ = 17.12 ± SE 0.48; n = 342); this is 2.93–3.98 times higher than all previous extrapolated estimates (Table 2). Circa 4,042 individuals (69%) live within protected areas and 1,820 (31%) live outside protected areas.
Mean group size was considerably higher in protected areas (μ = 20.57 ± SE 0.63; n = 190) compared to non-protected areas (μ = 12.80 ± SE 0.58; n = 152) (Fig. 3) and this difference was highly significant (Wilcoxon signed rank test: W = 6815, P < 0.05). The total number of adult females was 3,179 (54.21% of the total), with a mean of 9.29 ± SE 0.29 adult females per group (n = 342). The total number of adult males was 932 (15.89%), with a mean of 2.71 ± SE 0.08 per group (n = 342). These data demonstrate a ratio of 3.31 adult females to adult males across the species’ range (Fig. 4). However, the ratio differed significantly between protected and non-protected areas (χ2(340) = 3.39, P = < 0.05), with a significantly lower ratio outside protected areas. The total number of subadults/juveniles was 793 (13.52% of the total), with a mean of 2.32 ± SE 0.11 per group (n = 342), and the total number of infants was 958 (16.34%), with a mean of 2.80 ± SE 0.11 per group (n = 342), yielding ratios of 0.30 infants to adult females, and 0.25 subadults/juveniles to adult females. The ratio of infants to adult females did not differ between protected and non-protected areas (χ2(340) = 0.584, P = 0.449), nor did the ratio of subadults/juveniles to adult females (χ2(340) = 0.627, P = 0.427).

Fig. 3 Mean group size of P. kirkii in various protected and non-protected areas and habitat types.

Fig. 4 Ratios of (a) adult females to adult males and (b) infants to adult females, and subadults/juveniles to adult females of P. kirkii, for various key habitats, selected sites and levels of protection. Dotted lines represent mean ratio across all groups.
Discussion
Although there have been a number of previous estimates of the population size of P. kirkii (Table 1), we present the first systematically derived data on the total abundance and distribution of this primate. The conservation value of P. kirkii, the immediacy of threats posed by a growing human population and tourist sector, and the resources at our disposal guided our decision to perform a complete count by sweep census across Zanzibar's main island of Unguja. Having developed the methods and employed them successfully on two other primate species (Davenport et al., Reference Davenport, Mpunga and Machaga2007, Reference Davenport, De Luca, Jones, Mpunga, Machaga, Kitegile and Picton Phillipps2008), our aim was to ensure as precise a population assessment as possible. The complete count relies on locating and following every group (Davenport et al., Reference Davenport, De Luca, Jones, Mpunga, Machaga, Kitegile and Picton Phillipps2008). However, despite the considerable effort undertaken, we acknowledge the possibility that double-counting may have occurred as a result of occasional challenges associated with dense habitat, fission–fusion, sampling bias or group overlap. We also recognize potential problems associated with inter-observer consistency regarding the identification of age–sex categories. Nevertheless, the methods we employed and the training we provided were designed to minimize potential errors, and we aimed to provide one of the few demographic datasets for an entire primate species.
Although comparatively small for a primate population, a total population of 5,862 individuals is almost three times larger than the highest previous estimate for this species. The reasons behind this disparity are unclear. From its discovery to science in 1886 to the early 20th century P. kirkii was described as very rare and on the verge of extinction. A century later the total population was estimated to comprise 1,000–2,400 individuals. It is tempting to assume that these latter estimates (Table 1) were based on incomplete samples and insufficient knowledge of the species’ full extent of occurrence. However, it may also be the case that the former descriptions reflected the widespread forest clearance that took place across Zanzibar in the mid 19th century for clove plantations (Hazell, Reference Hazell2011). It is possible that after the collapse of the clove industry following the hurricane of 1872, and the market crash of the 1920s, forests and associated fauna began to recover. The assumption that P. kirkii was, and always has been, in decline may be incorrect, and the population may have been increasing. The data from Jozani–Chwaka Bay National Park, Uzi, and especially Masingini Forest Reserve, indicate that marked population growth of the species is possible even in conditions that are less than ideal (Struhsaker & Siex, Reference Struhsaker and Siex1998a, Reference Struhsaker and Siexb). Our count of 309 individuals in Masingini Forest Reserve is the latest point in an exponential increase (Fig. 5) for this small forest since P. kirkii was introduced there (Table 1). The current recruitment in Masingini is low, as reflected by a subadult/juvenile to adult female ratio of 0.13, compared to 0.74 in 1994 (Struhsaker & Siex, Reference Struhsaker and Siex1998a,Reference Struhsaker and Siexb), although this may reflect an isolated subpopulation that has now reached capacity. What is clear is that the red colobus is a more resilient and abundant animal than anyone since 1868 had thought.

Fig. 5 P. kirkii population increase in Masingini Forest Reserve (Fig. 1) during 1974–2014.
However, the species faces serious challenges and, with 5,862 individuals, P. kirkii is rare by most primate standards. In Tanzania, an important country for primate conservation (Davenport et al., Reference Davenport, Stanley, Sargis, De Luca, Mpunga, Machaga and Olson2006, Reference Davenport, Nowak and Perkin2014), it is the third most threatened diurnal primate, after R. kipunji (1,117 individuals; Davenport et al., Reference Davenport, De Luca, Jones, Mpunga, Machaga, Kitegile and Picton Phillipps2008) and Cercocebus sanjei (2,800–3,500 individuals; McCabe et al., Reference McCabe, Rovero, Fernández, Struhsaker and Butynskiin press); P. gordonorum is fourth, with 30,000–40,000 individuals (Rovero et al., Reference Rovero, Barelli, Butynski, Marshall and Struhsakerin press). Furthermore, mean recruitment/survivorship is low, indicating a population in decline across Unguja. Subadult/juvenile to adult female ratios as low as 0.25 are rare among all red colobus species that have been studied (15 samples from six taxa; Struhsaker, Reference Struhsaker2010), and the two populations with similar ratios were threatened by habitat loss or predation.
Tourism continues to have a negative effect on P. kirkii across Unguja, and recent hotel and residential developments have destroyed forest habitat and/or connectivity in Uroa, Mchangamle, Kiwengwa, Jambiani, Chwaka, Nungwi, Fumba and Michamvi. Meanwhile, demographic data indicate that mean group size is significantly smaller outside protected areas than within. Group size in red colobus species is determined by predation pressure, habitat quality and sociological dynamics (Struhsaker, Reference Struhsaker2010). Following the probable extinction of the Zanzibar leopard Panthera pardus pardus in the 1980s, people are the only predators of P. kirkii. Predation by people may be considerable, and hunting has been shown to result in smaller group sizes in P. tephrosceles (Stanford, Reference Stanford1998). Although the effect of predation tends to be less strong than that of habitat (Struhsaker, Reference Struhsaker2010), in Zanzibar at least, it appears to be significant. Nonetheless, large groups of red colobus usually occur in large forest blocks of good habitat, whereas smaller groups typically occur in small forest patches or degraded forest (Struhsaker, Reference Struhsaker2010). This is consistent with our finding significantly smaller group sizes outside protected areas, probably as a result of fragmentation, and reduced resources and food availability, as found in other colobines (Chapman et al., Reference Chapman, Chapman, Bjorndal and Onderdonk2002; Struhsaker et al., Reference Struhsaker, Marshall, Detwiler, Siex, Ehardt, Lisbjerg and Butynski2004), as well as higher levels of hunting by people. Barelli et al. (Reference Barelli, Albanese, Donati, Pindo, Dallago and Rovero2015) reported that α-diversity of gut microbiota in P. gordonorum in the Udzungwa Mountains was significantly higher in undisturbed forest. This variation may be associated with food plant diversity and may also influence the survival of P. kirkii.
Siex (Reference Siex2003) found the male to female sex ratio in P. kirkii in and around Jozani–Chwaka Bay National Park was high (1 : 4.6) and variable. In 1992–1993 the sex ratio in the shambas outside the Park was 6.9, much higher than inside; however, by 1999 this had declined to 3.8 as a result of population compression into the shamba from degraded forest, and male immigration (Siex, Reference Siex2003). Our data indicate a lower sex ratio of 1 : 3.31 across Unguja (and the species), as well as significantly lower ratios outside protected areas compared to inside. This overall ratio suggests that compression may not have been the cause of the decline in sex ratio, or at least not completely. Van Schaik & Hörstermann (Reference van Schaik and Hörstermann1994) argued that where predators are common there are more males and thus lower sex ratios. Our surveys showed the extent of human predation on P. kirkii, and the sex ratio may not only provide ecological evidence of this, but also show that it is much more prevalent, unsurprisingly, outside protected areas. The impact of mortality can be reflected more strongly in the ratio of adult females to subadults/juveniles than infants (Struhsaker, Reference Struhsaker2010), but like Siex (Reference Siex2003) we found no significant differences between these ratios, or between female to infant ratios inside and outside protected areas, although our data indicate there has been a 3.4–5.7 fold decline in recruitment in and around Jozani–Chwaka Bay National Park since 1992 (Siex & Struhsaker, Reference Siex and Struhsaker1999; Siex, Reference Siex2003).
Circa 85.5% of P. kirkii were located in forest (primary, secondary, forest edge and thicket), with 4.5% occurring in each of shamba and mangroves. Although the species is clearly capable of surviving, and even thriving, in degraded habitat, forest in some form is essential. Although mangroves may be a refuge for a few individuals, notably in Maji Mekundu, Ukongoroni and parts of Uzi, it does not appear to be an important or source habitat at the species level as postulated by Nowak & Lee (Reference Nowak and Lee2011).
It is likely that most of the groups and individuals outside protected areas will not survive in the long term as habitat is lost. This puts 1,820 individuals (the 31% of the total population that live outside protected areas) in jeopardy, with isolated groups in Kibele, western Uzi, Maji Mekundu, Mtende, Michamvi and the eastern coastal strip most at risk. The species has already been extirpated from Nungwi, Matemwe, Kichwele, Jendele and Dunga since the late 1990s. The proposed Jambiani–Muyuni Forest Reserve has only 103 individuals in nine groups and will therefore protect only a small portion (1.8%) of the total P. kirkii population. Nonetheless, greater protection for this area resulting from its gazettement as a Forest Reserve may facilitate P. kirkii population growth.
Nowak (Reference Nowak2007) reported that the Uzi/Vundwe population was at least twice the size of that in Kiwengwa. However, according to our data there are currently 2.7 times as many P. kirkii individuals in Kiwengwa as in Uzi/Vundwe. There is justification for improved management of Uzi/Vundwe (Nowak & Lee, Reference Nowak and Lee2011; Davenport et al., Reference Davenport, Nowak and Perkin2014), although the assertion that this is needed because mangrove-dwelling groups are a behaviourally and ecologically distinct subpopulation (Nowak & Lee, Reference Nowak and Lee2011) may now be open to debate. However, there is certainly a strong case for the gazettement of a new protected area to protect > 600 P. kirkii individuals and other biodiversity, covering southern Uzi, Vundwe and Mchangamle across Pete Inlet. Mchangamle offers greater potential for long-term viability, with extant habitat corridors to the north and south. Vundwe Islet is not officially inhabited, but during visits in April and August 2017 we found that migrant fishing camps there have expanded and become more permanent.
Hunting with dogs, guns and sometimes poison is a significant threat to wildlife. Piliocolobus kirkii and Cercopithecus mitis albogularis are both killed for meat for people and for dogs, and as a pest. This is despite traditional beliefs that kima punju (poison monkeys) are unfit for human or canine consumption. The practice is widespread, and monkey hunters were observed during the surveys in Kibele, Marumbi, Ufufuma, Mchangamle, Jozani and Kiwengwa. During 2007–2013 poisoned water was routinely left out to kill P. kirkii in mangroves on Uzi. The last known individual in Kichwele Forest Reserve was killed by hunters in 2010, and monkey carcasses are sold for USD 3–6 each.
The total AOO calculated as the sum of the occupied grid squares is 376 km2 and the total EOO (species range) is 428 km2 (i.e. 24.6 and 28% of the total land area of Unguja, respectively), although these figures could be smaller if calculated with grid cells smaller than 2 × 2 km. The occurrence of P. kirkii across a quarter of the island is in contrast to Kirk and Johnson's observations in 1884 and 1886, respectively, that the monkey was ‘lingering on in one clump of forest’ only (Forbes, Reference Forbes1894).
A taxon is considered to be threatened if the best available evidence indicates that it meets any one of a number of criteria (IUCN, 2017). P. kirkii was most recently assessed as Endangered based on criteria B1a,b(ii,iii,v) (Struhsaker & Siex, Reference Struhsaker and Siex2016); however, our data do not support this unequivocally, as there is no empirical evidence of ‘extreme fluctuations’ or a reduction in population size, even with hunting by humans. It could be argued that the population is increasing, especially within protected areas, which contain 69% of the global population. Nonetheless, low recruitment, forest loss, an increasing human population, and development all justify a projected reduction. The crucial factor, therefore, lies in a subjective assessment of whether the population is severely fragmented. If we adopt the precautionary principal and accept that it is, based on the isolation of several subpopulations, then we recommend a categorization of Endangered based on criteria B1a,b(i,ii,iii).
This survey did not include P. kirkii in Ngezi Forest Reserve on Pemba Island and we do not include them as part of the census. In 1973 c. 15 individuals were introduced to the island from Unguja (Silkiluwasha, Reference Silkiluwasha1981), and the latest reports are that 35–40 now survive there (Butynski & De Jong, Reference Butynski, De Jong and Soorae2011). Although it is possible that P. kirkii may have existed in Masingini and other sites on Unguja, this small population in Ngezi is definitely exotic and its conservation there is hard to justify, not least because of its possible impact on indigenous flora and fauna. We believe there is a case for the re-location of these individuals back to Unguja.
The results we present are surprising and they offer some grounds for optimism. Although Zanzibar's forests continue to be lost, and resources to manage them are meagre, income from well-managed primate and forest tourism can help. In 2016 Jozani–Chwaka Bay National Park accrued revenue of USD 334,700 (Tahir Abasi, pers. comm.), most of which was attributable to P. kirkii viewing. This could be further developed, with additional habituated groups in Kiwengwa, Masingini, Uzi or Jambiani–Muyuni, for example. We also recommend that P. kirkii be adopted by the Revolutionary Government of Zanzibar as the official national animal, as there can be few more charismatic and appropriate candidates. The persistence of Zanzibar's unique red colobus would be a fitting tribute to the efforts of the Government, as well as John Kirk, the man whose name the species bears and who accomplished so much for science and humanity alike.
Acknowledgements
All work was funded by the Wildlife Conservation Society (WCS). Research permission was granted by the Department of Forestry and Non-Renewable Natural Resources in Zanzibar. We thank Shaabani Iman, Ali Kassim Ali, Almasi Ramadhani, Hilali Akiba, Abasi Juma Mzee, Tahir Abasi, Lazaro Benedict, Sarah Markes and Rob Critchlow for their varied and considerable assistance. We also thank the Community Conservation Committees on Unguja for their support. Thomas Struhsaker and an anonymous referee provided helpful comments.
Author contributions
TRBD conceived the research, raised the funds, designed the methods, directed the fieldwork, visited all sites, analysed the data, and wrote the article. SAF, SPK and LUK coordinated with village authorities, led teams in the field, managed data collection and edited the text. LSF carried out GIS work and text editing. DWDL provided technical advice, oversaw project logistics and human resources, and carried out statistical analysis and edits.
Biographical sketches
Tim Davenport set up and directs the WCS Tanzania Program. His research interests are broad and include anything with applied conservation relevance. Said Fakih is a botanist, forester and assistant director of the WCS Zanzibar Program. Sylvanos Kimiti manages field teams in the Southern Highlands studying carnivores, primates and ungulates. Lydia Kleine is a biologist who spent 2010–2013 in Zanzibar, and currently works as a commercial salmon fisher in Alaska. Lara Foley manages the WCS Tarangire Elephant Project and uses GIS technology to study wildlife across the Tarangire ecosystem. Daniela De Luca is a carnivore biologist who specializes in carnivore and threatened species ecology, conservation and monitoring. She set up the Uganda mongoose project in 1990.









