This paper contains supplementary material that can be found online at http://journals.cambridge.org
Introduction
The Mediterranean and adjacent regions are considered among the most important refugial zones of the Tertiary relict flora in western Eurasia (Milne & Abbott, Reference Milne and Abbott2002; Milne, Reference Milne2006), including relict trees of the genera Aesculus, Juglans, Liquidambar, Pterocarya and Zelkova (Browicz, Reference Browicz1982). Most of these relicts have a restricted distribution and are at risk of extinction, often with only a few natural populations that survived the last glaciation in isolated areas or on Mediterranean islands (Quézel & Médail, Reference Quézel and Médail2003; Connor, Reference Connor2009; Garfì et al., Reference Garfì, Carimi, Pasta, Rühl and Trigila2011).
Zelkova abelicea (Lam.) Boiss. (Ulmaceae), a Tertiary relict tree endemic to the Greek island of Crete, is one of two European representatives of this otherwise Asiatic genus (Fineschi et al., Reference Fineschi, Anzidei, Cafasso, Cozzolino, Garfì and Pastorelli2002, Reference Fineschi, Cozzolino, Migliaccio and Vendramin2004; Kozlowski & Gratzfeld, Reference Kozlowski and Gratzfeld2013). The second Mediterranean species, Z. sicula, was discovered in 1991 in Sicily (Di Pasquale et al., Reference Di Pasquale, Garfì and Quézel1992). The genus has a particularly disjunct distribution. In addition to the two Mediterranean species, one species occurs in Transcaucasia (Z. carpinifolia) and three in eastern Asia (Z. serrata, Z. schneideriana and Z. sinica; Zheng-yi & Raven, Reference Zheng-yi and Raven2003; Denk & Grimm, Reference Denk and Grimm2005). The isolation of the east Asiatic and west Eurasian taxa most likely began in the Middle Miocene as a result of increasing aridity in central Asia. In western Eurasia Zelkova became restricted to refugia during the Pleistocene ice ages (Wang et al., Reference Wang, Ferguson, Zetter, Denk and Garfì2001). The establishment of a summer dry climate in the Mediterranean area during the Quaternary further contributed to the fragmentation of the range of the genus. Zelkova had already disappeared from continental Greece by the Middle Pleistocene, c. 400,000 BP, but persisted in central Italy until c. 30,000 BP (Follieri et al., Reference Follieri, Magri and Sadori1986; Van der Wiel & Wijmstra, Reference Van der Wiel and Wijmstra1987).
Relict species such as Z. abelicea provide a unique opportunity to understand past and recent biogeographical and evolutionary processes. The species is one of the most notable Tertiary relict trees of the Mediterranean region because it is found at higher altitudes than other relict trees such as Liquidambar orientalis and Phoenix theophrasti. Moreover, it is one of the few tree species endemic to the eastern Mediterranean. For these reasons the species is of scientific and conservation interest (Kozlowski & Gratzfeld, Reference Kozlowski and Gratzfeld2013).
All four of the main Cretan mountain ranges hold populations of Z. abelicea: the Levka Ori, Psiloritis, Dhikti and Thripiti Mountains (Jahn & Schönfelder, Reference Jahn and Schönfelder1995; Rackham & Moody, Reference Rackham and Moody1996; Fazan et al., Reference Fazan, Stoffel, Frey, Pirintsos and Kozlowski2012; Kozlowski & Gratzfeld, Reference Kozlowski and Gratzfeld2013). Z. abelicea is found in open mountain forests, where it coexists with Acer sempervirens, Quercus coccifera and occasionally Cupressus sempervirens (Egli, Reference Egli1997). The species primarily occupies north-facing slopes and areas around dolines, although it can also grow in or near rocky river beds and gullies, where moisture tends to remain near the surface during the dry summer (Søndergaard & Egli, Reference Søndergaard and Egli2006; Fazan et al., Reference Fazan, Stoffel, Frey, Pirintsos and Kozlowski2012).
Despite its emblematic status among conservationists and dendrologists (de Spoelberch, Reference De Spoelberch1993), Z. abelicea has never been studied or monitored comprehensively throughout its range (Kozlowski et al., Reference Kozlowski, Gibbs, Huan, Frey and Gratzfeld2012a).
Our aims were to (1) update current knowledge on the historical and recent distribution of Z. abelicea, (2) gather data to improve our understanding of the population structure of Z. abelicea and the threats that it faces, based on study plots in selected populations across its range, and (3) re-evaluate the conservation status of the species to contribute to the design of conservation measures based on new evidence. This study is the first to examine the populations of the species in all four mountain ranges of Crete.
Study area
The island of Crete in the southern Aegean Sea (Fig. 1) is one of the largest islands of the Mediterranean basin (8,729 km2), with a landscape dominated by karst mountains. Crete has a typical Mediterranean climate, with hot, dry summers and cool, wet winters. At higher elevations annual precipitation can exceed 2,000 mm and snowfall is not unusual during winter, although snow accumulates only above 1,400 m, where it may persist until May (Rackham & Moody, Reference Rackham and Moody1996). Precipitation is higher in the western part of the island, and moisture from winter precipitation may be stored locally in the soil throughout the summer (Egli, Reference Egli1997). Crete is a remnant of an island arc that once connected Greece to Turkey and it has been separated from the mainland for at least 5 million years (Schüle, Reference Schüle1993). This isolation is consistent with its significant value as a floristic refugium because > 10% of its 1,700 plant species are endemic to the island (Turland et al., Reference Turland, Chilton and Press1993).
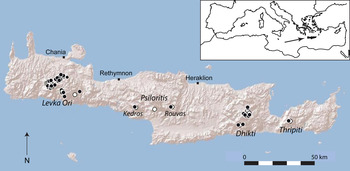
Fig. 1 Historical and recent distribution of Zelkova abelicea. Black circles: recently observed and/or described populations (1993–2010). White circles: presumably extinct populations known from literature and herbaria (1700–1992).
Methods
We established the historical and recent distribution of Z. abelicea using several sources. As well as carrying out detailed field surveys we reviewed literature and visited herbaria and collections with the most comprehensive holdings of specimens from Crete, including Geneva, Lyon, Montpellier, Paris, Fribourg, Berlin and Kew (Holmgren & Holmgren, Reference Holmgren and Holmgren1998). We defined as historical all natural occurrences of Z. abelicea recorded between the first known herbarium records (AD 1700) and the first detailed publication of Egli (Reference Egli1993), which contains the most comprehensive modern data source for the abundance and distribution of Z. abelicea. Thus, the recent occurrences include all populations observed and described between 1993 and 2010.
We carried out an additional in situ survey of selected populations of Z. abelicea in summer 2010. We visited 14 plots in all four mountain regions with known Zelkova populations (Table 1, Supplementary Fig. S1). Eight plots were selected in the Levka Ori Mountains and three in the Dhikti Mountains. The survey in the Thripiti Mountains was restricted to the only known population of Z. abelicea. In the Psiloritis Mountains the survey was restricted to the two known populations, one in the Rouvas Forest and one on Mount Kedros. In small populations (areas of < 1,000 m2) the plot included the entire stand, and for larger populations with less clearly defined limits, plots of various sizes (500 to > 20,000 m2) were used (Supplementary Table S1). In larger populations two or more plots were surveyed to ensure that any environmental heterogeneity was covered by the sampling.
Table 1 The 14 plots where Zelkova abelicea was surveyed (Supplementary Fig. S1), with altitude, area and the intensity of occurrence of threats to the species: overgrazing by livestock, drought and other forms of water stress, wood exploitation (e.g. pollarding, cutting of branches), soil erosion and/or rockfall, geographical isolation, and signs of recent fires. For conservation reasons, the exact coordinates of the study plots are not provided.

* −, negligible or not observed; +, relatively low; ++, strong; +++, extreme
In each study plot we determined the geographical coordinates and altitude, assessed observed and potential threats using a standardized form, counted the number of Z. abelicea individuals and noted the presence or absence of fruiting trees. Trees were recorded as being in one of three categories: (1) trees that had a fully developed crown, that had escaped browsing except for the lowest branches and that produced fruit, (2) wholly browsed trees of shrubby dwarfed habit that did not produce fruit, and (3) an intermediate category with browsed lower branches and unbrowsed upper branches that were not yet producing fruit. This classification does not incorporate age but rather attempts to represent the vitality of a given tree (Fazan et al., Reference Fazan, Stoffel, Frey, Pirintsos and Kozlowski2012).
The geographical extent, with coordinates, of all known stands of Z. abelicea was mapped, and the calculation of population sizes by Egli (Reference Egli1993 & unpubl. data) and in this work was completed during field surveys in summer 2010. The extent of occurrence is defined as the area contained within the shortest continuous imaginary boundary that can be drawn to encompass all the known, inferred or projected sites of present occurrence of a taxon, excluding cases of vagrancy, and the area of occupancy is defined as the area within the extent of occurrence that is occupied by a taxon, excluding cases of vagrancy. Both of these areas, the latter based on a cell size of 2 × 2 km, were calculated according to IUCN (2010) using the online tool developed by the Royal Botanic Gardens, Kew (GeoCAT, 2012).
Results
Historical and recent distribution of Z. abelicea
Fig. 1 illustrates the distribution of Z. abelicea during 1700–2010 on the basis of historical literature, herbarium records and recent observations. The occurrence of Z. abelicea in the Levka Ori was known to the scientific community at the beginning of the 18th century (Supplementary Table S2), in the Dhikti Mountains in the second half of the 19th century, and on Mount Kedros and in the Rouvas Forest in the first half of the 20th century. The small, isolated population in the Thripiti Mountains was discovered during the second half of the 20th century.
The recent distribution of Z. abelicea (1993–2010) concurs almost completely with the historical occurrences, with no dramatic shifts in distribution during the previous 300 years. However, several isolated populations, primarily in the Levka Ori and Psiloritis Mountains, appear to have become extinct during the second half of the 20th century (Fig. 1, Supplementary Table S3). We did not discover any new populations during field surveys.
There are 42 known populations of Z. abelicea (Fig. 1, Supplementary Table S3), 30 in the Levka Ori Mountains and nine in the Dhikti Mountains. There are two populations in the Psiloritis Mountains, one on the northern slopes of Mount Kedros and the other in the Rouvas Forest. Only one small population is known in the Thripiti Mountains.
The species is found up to altitudes of almost 1,800 m in the Levka Ori Mountains, up to c. 1,600 m in the Dhikti Mountains, and the populations in the Psiloritis and Thripiti Mountains are at 1,150–1,350 m (Table 1, Supplementary Table S3).
The extent of occurrence of the species is 2,094 km2 and the area of occupancy 64 km2. Most of the area of occupancy is located in the Levka Ori Mountains (80%), with 19% in the Dhikti Mountains and 0.3% in each of the other three populations (Supplementary Table S4).
Population structure and species abundance
In all 14 surveyed plots most individuals were dwarf and severely browsed (Fig. 2, Supplementary Table S1). In the Psiloritis, Dhikti and Thripiti Mountains 90–100% of the trees were dwarf. Only in the Levka Ori Mountains did we find populations with fewer heavily browsed trees (e.g. OMA2: 53%; OMA1: 56%). Between 0 and 15% of individuals were in the intermediate category, and in most study plots 0–17% of trees were normally developed, with only two plots in the Levka Ori Mountains having higher levels (OMA1: 38%; OMA2: 32%). Several study plots had abnormally developed trees (e.g. STA, NIA). In LAS3 only severely browsed individuals were present. We observed that only fully developed trees produced fruit. Fruiting was observed in only half of the study plots (Fig. 2).

Fig. 2 Population structure of Z. abelicea in the 14 study plots (Table 1).
A total of 6,007 individuals of Z. abelicea were counted in the 14 study plots (Supplementary Table S1). Of these, 91% (5,459) were severely browsed and dwarf and only 5% (320) had a normally developed habit. Four percent (228 individuals) were intermediate trees, with branches recently freed from browsing.
The density of trees was relatively high (1,328 ha−1; Supplementary Table S1). However, because most trees were severely browsed and dwarf, the mean density of intermediate and large trees was low (39.9 and 27.5 ha−1, respectively). Generally, density was higher in severely browsed populations (Supplementary Table S1). These populations were characterized by dwarf individuals, most likely of clonal origin. Populations with higher percentages of large trees were less dense (OMA2: 265 ha−1; OMA1: 448 ha−1).
The total area of all mapped Z. abelicea populations was 884.7 ha (Supplementary Table S4). Given the mean density of Z. abelicea (Supplementary Table S1), it is possible to estimate the total number of individuals of Z. abelicea. There could be a total of > 1,000,000 dwarf Z. abelicea individuals in the four mountain ranges studied. Although large trees constitute only a small proportion of the Z. abelicea populations, there could be as many as 20,000 normally developed and fruiting individuals (the majority in the Levka Ori Mountains).
Threats
Table 1 and Fig. 2 show that the most severe pressure on all investigated populations is overgrazing and browsing by livestock. This is followed by soil erosion, which is clearly correlated with intensive trampling and grazing and was observed in almost 60% of the plots. The potential influence of drought during the summer months is difficult to establish, although symptoms of water stress (e.g. dead branches without signs of browsing, dry and brownish leaves during the vegetative period) were observed in several study plots (e.g. ELI, PSI and LAS3). Pollarding and other forms of wood utilization appear to be minimal and were observed only in plots with a relatively large number of normally developed trees (e.g. in the Levka Ori Mountains). Fires are a significant source of disturbance to the vegetation of Crete, and signs of recent burning were observed in > 40% of the study plots. Finally, the population in the Thripiti Mountains, the two distant populations in the Psiloritis Mountains and several populations in the Dhikti and Levka Ori (e.g. ELI, NIA) are geographically isolated (most likely without gene flow between other populations).
Discussion
Range fragmentation and the importance of disjunct populations
Our data suggest that the fragmentation of the distribution of Z. abelicea (Fig. 1) occurred prior to the modern botanical exploration of Crete c. 300 years ago. Although Crete has a long history of human presence and disturbance (Cowling et al., Reference Cowling, Rundel, Lamont, Kalin Arroyo and Arianoutsou1996; Atherden & Hall, Reference Atherden and Hall1999) it is not clear whether this fragmented distribution is a result of anthropogenic impacts, including habitat transformation. Whether or not Crete was dominated by continuous woodland before the arrival of man is subject to debate (Schüle, Reference Schüle1993; Fielding & Turland, Reference Fielding and Turland2005). Grazing and browsing are known to have occurred on the island long before human inhabitants arrived, as Crete was inhabited during the Pleistocene by large herbivores, including species of deer Candiacervus cretensis, dwarf elephant Palaeoloxodon chaniensis and hippopotamus Hippopotamus creutzburgi (Rackham & Moody, Reference Rackham and Moody1996), which required large, prairie-like feeding grounds. It is therefore possible that the range of Z. abelicea always consisted of patches, even if these shifted to higher or lower altitudes and expanded or contracted over time. Z. abelicea is a temperate Holarctic element of the Cretan flora (Thompson, Reference Thompson2005), occurring exclusively at sites with relatively high water abundance: at higher elevations, on northern slopes and in or around dolines (Søndergaard & Egli, Reference Søndergaard and Egli2006). As a result of dramatic climatic changes associated with Pleistocene glacial cycles, Z. abelicea either migrated to or survived only in these most suitable sites after the end of the Ice Age.
Whatever the causes of this fragmentation (a complex colonization history, climate change, browsing by large Pleistocene herbivores or more recent agricultural practices), gene flow between remote populations is improbable because of the limited dispersal capacity of seeds (Hoshino, Reference Hoshino1990), as is the case for all Zelkova species. Thus, the species does not appear to have a metapopulation structure. The interaction of spatially discrete populations appears to be extremely limited. Recolonization through a colonization–extinction dynamic in small geographically isolated populations is not expected to compensate for extinctions (Hanski, Reference Hanski1998). In addition, the relictual nature of Z. abelicea suggests that the extant populations have been isolated from each other for a long time.
Altitudinal range
Z. abelicea occurs at high elevations in supra- and oro-Mediterranean mixed discontinuous woody stands dominated by evergreen and deciduous trees (Sarlis, Reference Sarlis1987; Søndergaard & Egli, Reference Søndergaard and Egli2006). Palaeobotanical data indicate that the genus Zelkova belongs to a hygro-meso-thermophilous floristic unit that occurred abundantly throughout Europe before the last glacial age (Di Pasquale et al., Reference Di Pasquale, Garfì and Quézel1992; Quézel & Médail, Reference Quézel and Médail2003). The Hyrcanian–Euxinian forests of the Transcaucasian region are the last remnants of this flora in western Eurasia (Browicz, Reference Browicz1989). Z. carpinifolia thrives there at 100–600 m, although it can grow at higher altitudes (e.g. at 1,200 m in Talysh, Azerbaijan), where it occurs only in a shrubby form (Kvavadze & Connor, Reference Kvavadze and Connor2005). In China the three eastern Asian Zelkova species occur in forests that are functionally similar to each other, and they usually grow to 30–35 m in height and occur mostly at 200–2,500 m. The Mediterranean species Z. sicula, endemic to Sicily, is known only from two relict populations at c. 350 and 500 m. The species grows in thermophilous evergreen and semideciduous oak wood communities in a typical thermo/meso-Mediterranean bioclimate. However, this may not be the optimum environment for the species (Garfì et al., Reference Garfì, Carimi, Pasta, Rühl and Trigila2011). It is therefore assumed that both Mediterranean Zelkova species survived the Ice Age at lower altitudes and/or latitudes. After the Holocene climate warming, migration of Z. abelicea towards more suitable habitats could have been facilitated by effective, although reduced, sexual propagation. Z. sicula, however, reproduces exclusively through vegetative multiplication (Garfì et al., Reference Garfì, Barbero and Tessier2002).
Dwarf trees and asymmetric population structure
Of the > 6,000 Z. abelicea surveyed only 320 (c. 5%) had a well-developed main stem and crown and usually produced flowers and fruits. Our findings show that, with a few exceptions in the Levka Ori Mountains, the populations of Z. abelicea are dominated by dwarf individuals. This asymmetry in the population structure is especially pronounced in relatively small and isolated populations (e.g. in Thripiti, where there are no normally developed trees) and should be considered in any conservation planning. Most previous conservation efforts have focused on well-developed trees, which are visible elements of the landscape (Fazan et al., Reference Fazan, Stoffel, Frey, Pirintsos and Kozlowski2012).
There are many potential causes for the dwarf habit. For Z. abelicea the most important are intensive browsing and water stress (Fazan et al., Reference Fazan, Stoffel, Frey, Pirintsos and Kozlowski2012), along with the further constraining factors of fire, low nutrient availability, and coppicing and pollarding (Rackham & Moody, Reference Rackham and Moody1996). Generally, the intensity of browsing and grazing in populations of Z. abelicea is unusual in comparison with other Zelkova species. Both known populations of Z. sicula and the majority of those of Z. carpinifolia are browsed exclusively by cows. On Crete, the principal and omnipresent domestic herbivores are goats and sheep, whose grazing and browsing are more intense and have a major impact on tree habit and vigour.
Conclusions and recommended priority actions
Z. abelicea is an atypical member of its genus for the following reasons: (1) it can grow at the timberline, (2) it is significantly affected by ovine and caprine (rather than bovine) grazing pressure, (3) it shows a pronounced dimorphism of individuals (dwarf and normally developed trees), and (4) it has an asymmetric population structure dominated by dwarf trees (Kozlowski & Gratzfeld, Reference Kozlowski and Gratzfeld2013). Moreover, Z. abelicea is the only member of the genus with wax ornamentation on the leaf surface, which is thought to be an adaptation to the dry summer of the Mediterranean climate (Wang et al., Reference Wang, Ferguson, Zetter, Denk and Garfì2001; Denk & Grimm, Reference Denk and Grimm2005). It is likely that the species escaped historical extinction because of the complex topography of Crete and the suitable climatic conditions (e.g. high precipitation) in the island's mountain regions.
Given the fragmented spatial pattern of the limited geographical range of Z. abelicea (with an extent of occurrence < 5,000 km2 and an area of occupancy < 500 km2) and the characteristics of the species, we conclude that Z. abelicea is a threatened species (IUCN category Endangered, EN). The formal evaluation according to the IUCN Red List Categories and Criteria is Blab(iii) + 2ab(iii). The results described in this study were used in the most recent IUCN status assessment of the species (Kozlowski et al., Reference Kozlowski, Frey, Fazan, Egli and Pirintsos2012b).
An action plan and a series of workshops dedicated to the conservation of Z. abelicea are in preparation. We recommend the following priority actions in collaboration with the local authorities:
In situ conservation of the species' entire range
The fragmentation of Z. abelicea populations requires that each mountain massif where the species occurs should represent a separate conservation unit, with locally adapted conservation strategies. Core populations of immediate conservation concern, identified on the basis of geographic isolation, area covered and browsing pressure, include the sole population in the Thripiti Mountains and the Mount Kedros and Rouvas Forest populations in the Psiloritis range. Each of these isolated, and in some cases small, populations is at risk of being lost as a result of an accidental, deliberate or stochastic event (e.g. fire, road construction, drought). Effective methods and measures to limit and/or completely prevent livestock grazing need to be developed in close collaboration with shepherds and other local stakeholders and accompanied by long-term scientific surveys to monitor progress and allow adaptive management.
Enhance ex situ conservation
A major coordinated effort is required to establish viable, representative and well-documented collections at botanic gardens, arboreta and other affiliated institutions in collaboration with forest services in Greece, and especially Crete. The Z. abelicea collections cultivated in botanic gardens almost exclusively originate from the Levka Ori region (the most accessible area and the best-known occurrence of the species), therefore new collections based on plants from all other regions would significantly enhance the ex situ conservation value. Additionally, as the species is not present in any international seed bank, new seed collections should be undertaken. In particular, this should include collections from the small, highly isolated and threatened populations of Psiloritis, Dhikti and Thripiti, from where plant material has rarely, if ever, been collected for ex situ cultivation.
Foster public awareness of national botanical riches
Greece is endowed with a diverse flora, including unique, endemic and relict species such as Z. abelicea, and there is enormous potential to raise public awareness of this national biological wealth. Botanic gardens, especially in or near major urban centres, are ideal venues for developing a range of environmental campaigns and outreach activities, supported by educational materials and interactive exhibitions.
Acknowledgements
We thank Benoît Clément and Susanne Bollinger, Botanical Garden of the University of Fribourg (Switzerland), Emanuel Gerber and André Fasel, Natural History Museum Fribourg (Switzerland), Vaios Kalogrias (University of Crete) and Yann Marbach for their assistance during fieldwork and manuscript preparation. We are indebted to Fondation Franklinia for its generous support for this study, and to Douglas Gibbs (Botanic Gardens Conservation International) and Melanie Bilz (IUCN). Permission to investigate Z. abelicea populations was granted by the Ministry of the Environment, General Directorate of Forests, Department of Aesthetic Forests, National Parks and Wildlife Management, Athens, Greece (199076/1843).
Biographical sketches
Gregor Kozlowski's primary research interests are the conservation biology and biogeography of relict and endemic organisms. David Frey studies endemic taxa. Laurence Fazan is a geographer with research interests in the dendrochronology of relict trees in the Mediterranean. Bernhard Egli has specialist knowledge of doline soils and the vegetation of the mountains of Crete. Sébastien Bétrisey is studying the biogeography and conservation of Tertiary relict trees of south-west Eurasia. Joachim Gratzfeld specializes in the interface between people and biodiversity conservation. Giuseppe Garfì is a forest ecologist working on the biology and conservation of the woody plants of the Mediterranean; he discovered Z. sicula. Stergios Pirintsos is interested in the ecology and management of terrestrial ecosystems and in the ecology of rare and endemic plant species.





