Introduction
Knowledge of the geographical range of species and their abundance across this range is important for designing and prioritizing conservation and management activities (Wallace et al., Reference Wallace, DiMatteo, Hurley, Finkbeiner, Bolten and Chaloupka2010). Examining gaps in knowledge of a species’ distribution may uncover populations that are of regional, global, genetic or ecological importance. Estimating the size of a population provides an overview of the risk of extinction or loss (Gerrodette & Taylor, Reference Gerrodette, Taylor, Eckert, Bjorndal, Abreu-Grobois and Donnelly1999), given that small populations are more likely to disappear than large ones.
Despite numerous studies on the distribution and status of sea turtles, we still lack knowledge about certain populations (Mazaris et al., Reference Mazaris, Almpanidou, Wallace, Pantis and Scofield2014), especially in regions for which few data are available. Research on and conservation of sea turtles are relatively recent undertakings along the Atlantic coast of Africa (Formia et al., Reference Formia, Tiwari, Fretey and Billes2003) compared to the presence of long-term projects in the western Atlantic (e.g. Marcovaldi & Marcovaldi, Reference Marcovaldi and Marcovaldi1999; Troëng & Rankin, Reference Troëng and Rankin2005; Ehrhart et al., Reference Ehrhart, Redfoot, Bagley and Mansfield2014). Gaps remain in our knowledge and understanding of the status and ecology of sea turtles in the eastern Atlantic (e.g. leatherback turtles Dermochelys coriacea in the eastern Atlantic are categorized as Data Deficient on the IUCN Red List; Tiwari et al., Reference Tiwari, Wallace and Girondot2013), but increasing information both in the grey and scientific literature has highlighted conservation priorities and significant threats both on land and at sea (Witt et al., Reference Witt, Baert, Broderick, Formia, Fretey and Gibudi2009; Metcalfe et al., Reference Metcalfe, Agamboué, Augowet, Boussamba, Cardiec and Fay2015; Agyekumhene & Kouerey Oliwina, Reference Agyekumhene and Kouerey Oliwina2018).
In Angola, only sporadic and non-systematic surveys of the coast were conducted prior to the 1980s (Bocage, Reference Bocage1895; Monard, Reference Monard1937; Brongersma, Reference Brongersma1961; Hughes et al., Reference Hughes, Huntley and Wearne1973; Huntley, Reference Huntley1974), when more regular surveys were implemented (Hughes, Reference Hughes and Bjorndal1982a; Carr & Carr, Reference Carr and Carr1983, Reference Carr and Carr1991; Pires, Reference Pires1985; Afonso, Reference Afonso1987). This was followed by a period during which there was little research on sea turtles, until the early 2000s when the Kitabanga Project began regular and systematic surveys in the Palmeirinhas region (Fig. 1). By this time nesting numbers had decreased by > 70%, from a mean of 75 nests/km in 1985 to a mean of 21 nests/km in 2003 (Morais et al., Reference Morais, Andrade and Afonso2004). At the end of 2010, the Kitabanga Project initiated surveys along other parts of the coast and established permanent monitoring sites at a range of latitudes (Morais, Reference Morais2015, Reference Morais2016, Reference Morais2017, Reference Morais2018, Reference Morais2019, Reference Morais2020).
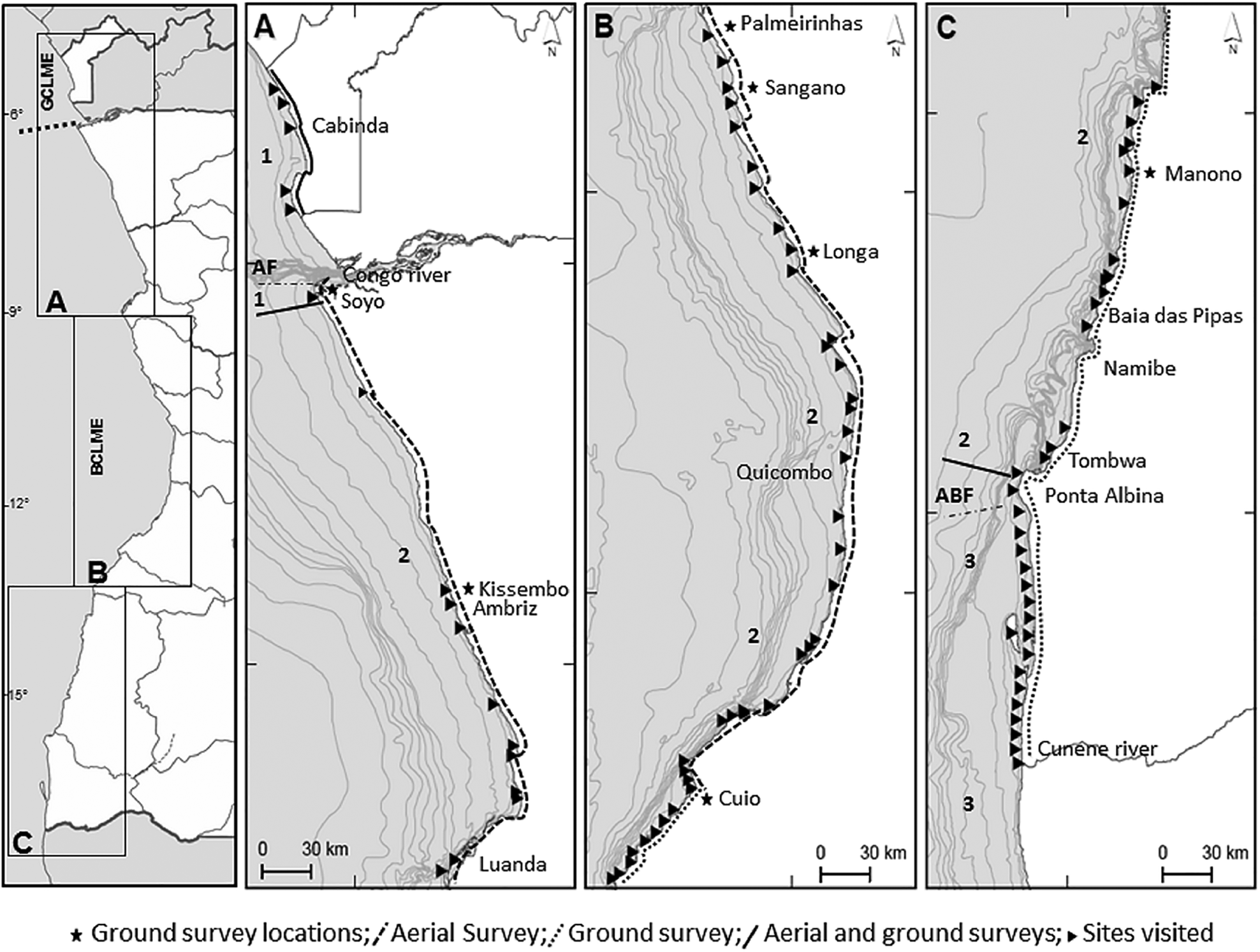
Fig. 1 The Angolan coastline and the edge of the continental shelf, indicating the locations of the seven permanent monitoring sites (Soyo, Kissembo, Palmeirinhas, Sangano, Longa, Cuio, Manono), paths of aerial and/or ground surveys, and all visited beaches (1, Southern Ecoregion of Gulf of Guinea; 2, Angolan Ecoregion; 3, Namibe Ecoregion; GCLME, Guinea Current Large Marine Ecosystem; BCLME, Benguela Current Large Marine Ecosystem; AF, Angolan Front; ABF, Angolan Benguela Front).
The coast of Angola is considered to be the southernmost range for nesting sea turtles in the eastern Atlantic (Carr, Reference Carr1957; Brongersma, Reference Brongersma and Bjorndal1982; Fretey, Reference Fretey2001). Studies and reports of sea turtles in Angola indicate the presence of five species: the olive ridley Lepidochelys olivacea, leatherback, green Chelonia mydas, hawksbill Eretmochelys imbricata and loggerhead Caretta caretta, of which only the first three species are known to nest in Angola (Bocage, Reference Bocage1895; Monard, Reference Monard1937; Brongersma, Reference Brongersma1961, Reference Brongersma and Bjorndal1982; Hughes et al., Reference Hughes, Huntley and Wearne1973; Huntley, Reference Huntley1974; Hughes, Reference Hughes and Bjorndal1982b; Carr & Carr, Reference Carr and Carr1983, Reference Carr and Carr1991; Pires, Reference Pires1985; Afonso, Reference Afonso1987, Fretey, Reference Fretey2001; Morais et al., Reference Morais, Andrade and Afonso2004; Buza, Reference Buza2005; CABGOC, 2006; Morais et al., Reference Morais, Torres and Martins2006; Weir et al., Reference Weir, Ron, Morais and Duarte2007; Morais, Reference Morais2008, Reference Morais2009, Reference Morais2011, Reference Morais2012a, Reference Morais2012b, Reference Morais2013, Reference Morais2014, Reference Morais2015, Reference Morais2016, Reference Morais2017, Reference Morais2018, Reference Morais2019; Ferreira et al., Reference Ferreira, Nogueira and Formia2009). Designing targeted conservation actions along this coastline requires an understanding of the spatial and temporal distribution of the sea turtle species nesting in the country.
The olive ridley is the most abundant sea turtle species in Angola, and is the focus of this study. This species exhibits three reproductive behaviours: arribada or synchronized mass nesting, dispersed or solitary nesting, and a mixed strategy (Kalb, Reference Kalb1999; Bernardo & Plotkin, Reference Bernardo, Plotkin and Plotkin2007; Fonseca et al., Reference Fonseca, Villachica, Matarrita, Argüello, Orrego, Quirós, Tucker, Belskis, Panagopoulou, Rees, Frick and Williams2013). In the western Atlantic, small-scale arribadas occur in Suriname and French Guiana (NMFS & USFWS, 2014), and there is a large solitary nesting population in Brazil (Silva et al., Reference Silva, de Castilhos, Lopez and Barata2007). In the eastern Atlantic, no arribadas have been reported, but nesting occurs along the Atlantic coast of Africa, with Gabon hosting the largest known olive ridley nesting population in the Atlantic (Metcalfe et al., Reference Metcalfe, Agamboué, Augowet, Boussamba, Cardiec and Fay2015).
Study area
The 1,650 km Angolan coast includes c. 1,400 km of sandy beaches (Fig. 1) and three Marine Ecoregions (Spalding et al., Reference Spalding, Fox, Allen, Davidson, Ferdaña and Finlayson2007): the Southern Ecoregion of the Gulf of Guinea (the entire coast of Cabinda southwards to approximately the Congo River), the Angolan Ecoregion (from the southern limit of the Southern Ecoregion to Tombwa) and the Namibe Ecoregion (Tombwa to the south of Angola; Fig. 1). The two northern regions have a tropical/subtropical climate and the Namibe Ecoregion has a temperate climate. As a result of the confluence of three major currents, the Benguela Current from the south, the South Equatorial Current that influences the northern coast, and the Current of Angola that displaces warm equatorial waters southwards, the coast of Angola is complex and dynamic. In addition, the South Equatorial Countercurrent and the Submarine Equatorial Current promote vertical movement of water. The discharge and currents from the Congo River have impacts up to 600 km from the coast (URS Greiner Woodward Clyde, 2000; CSIR Environmentek, 2001; Total Fina ELF, 2002; Morant, Reference Morant2003; ERM, Reference nvironmental, esourses and anagement2004).
The sea surface temperature decreases with increasing latitude and is influenced by the currents. In the north, between Cabinda and the mouth of the Congo River, mean sea surface temperatures vary between 28 °C in the hottest month (March) and 20 °C in the coldest months (July–August). Between the mouth of the Congo River and Baía Farta mean sea surface temperature is 18–23 °C. South of the Cunene River, minimum temperatures can reach 14 °C (Morais et al., Reference Morais, Torres and Martins2006). The dynamic complexity of the climate, currents and variation in sea surface temperature influence the spatial and temporal distribution of sea turtle nesting.
Methods
Aerial surveys
Aerial surveys have been increasingly used to monitor the presence and distribution of nesting turtles and population size (Pritchard, Reference Pritchard1982; Schroeder & Murphy, Reference Schroeder, Murphy, Eckert, Bjorndal, Abreu-Grobois and Donnelly1999; McGowan et al., Reference McGowan, Broderick, Frett, Gore, Hastings and Pickering2008; Witt et al., Reference Witt, Baert, Broderick, Formia, Fretey and Gibudi2009). We used aerial surveys to determine the spatial distribution of nesting. During 2011–2015, aerial surveys were conducted at the beginning of February each year, just after the peak in nesting at all sites, using an adaptation of the method used by Carr & Carr (Reference Carr and Carr1991) for surveys of the Angolan coast. The surveys were from the Congo River region (Soyo) to the Cuio region, with the exception of the surveys in 2011 and 2013 when the southern limit was Lobito (Fig. 1). The surveys were conducted during 06.30–10.00 over 2 consecutive days. On the first day, we surveyed the coastline between Luanda and Soyo, and on the second day between Luanda and the southern limit of Lobito or Cuio, covering a total coastline of 960 and 1,060 km, respectively. We used Aluete III and Bell 212 helicopters, flown at an altitude of 20–30 m and in some cases at 15 m, at a speed of 45–50 knots (sometimes at 40 knots in areas of high density). The use of helicopters facilitated slow flights at low altitudes and thus high accuracy of track counts and species identification using Prtichard & Mortimer's (Reference Pritchard, Mortimer, Eckert, Bjorndal, Abreu-Grobois and Donnelly1999) description of tracks; helicopters also provided the flexibility to fly over some high-density sections of beach again if the data needed to be double-checked. All flights had three observers on board, who recorded the locations of all nesting activities along the coast (tracks and nests, by species) with two GPS devices. The nest count data for olive ridley turtles were mapped for each year with ArcGIS 9.1 (Esri, Redlands, USA) using contiguous coastal sectors of 20 km. Non-nesting emergences (i.e. emergences without any evidence of nesting) were not included. Aerial identification of species’ tracks was confirmed with survey data collected at the Kitabanga Project's regular monitoring beaches. The aerial survey nest counts, which represent nests laid during the previous week (the period over which tracks are visible), were used only to determine the distribution of olive ridley turtle nesting along the coastline and where to focus monitoring efforts.
Beach surveys
For logistical reasons it was not possible to conduct aerial surveys south of Cuio and therefore ground surveys were conducted during 2012–2015 between Cuio and the Cunene River (Fig. 1) to record nesting evidence (tracks, nests, hatchlings, dead turtles). Between Cuio and the city of Namibe, the majority of the pocket beaches (i.e. small beaches isolated between two headlands) were visited. Between the city of Namibe and the mouth of the Cunene River at the border with Namibia, 250 km of coastline were surveyed by driving along the beach. In northern Angola, nesting records from surveys of the Cabinda coast are available for the 2007–2008 nesting season (Morais, Reference Morais2008).
Thus, of the 1,650 km Angolan coastline, 1,410 km were covered by aerial and/or ground surveys. The remaining 240 km of coastline between Cuio and Namibe that could not be surveyed are characterized by steep cliffs often without a sandy beach or with pocket beaches that are difficult to access by land. An aerial survey of this coastline was not possible because of the logistical difficulties of refuelling.
During 2011–2020, the Kitabanga Project expanded its permanent beach monitoring sites from three (22.6 km) to seven (53.9 km) to monitor nesting at different latitudes (Table 1). Together these beaches cover 3.3% of the coastline of Angola (Fig. 1).
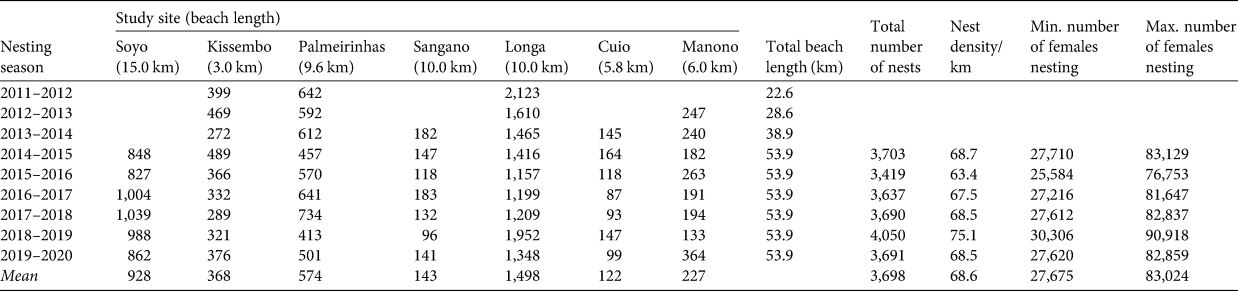
Table 1 Number of nests of the olive ridley sea turtle Lepidochelys olivacea at each of the seven permanent study sites along the coast of Angola (Fig. 1) during 2011–2020, total beach length and total number of nests, nest density/km, and estimated minimum and maximum number of females nesting during 2014–2020 (the years in which all seven beaches were surveyed). The minimum and maximum number of females nesting were estimated respectively from nest density × 1,210 km/3 and nest density × 1,210 km/1, because each female produces 1–3 clutches (see text for details).
In addition to these surveys, we contacted fishers and other local residents at all the beaches visited to obtain information on the spatial and temporal distribution of nests and captures in fisheries. These informal interviews were considered as complementary analysis (SWOT Scientific Advisory Board, Reference Scientific Advisory Board2011). This is important when the study area is extensive, time is limited and site visits cannot necessarily coincide with the biological cycle of the species studied.
Seasonality, density and estimation of nesting females
At the seven permanent monitoring sites all nesting activity during 2011/2012–2019/2020 (nine seasons) was recorded on a daily basis during morning surveys, which began at 05.00, during 1 September–30 April and weekly outside this period. Nightly surveys were carried out on these beaches during the same period, during 20.00–02.00, to identify and confirm species. These data were used to determine the temporal distribution of nests and nesting density.
The temporal distribution of nesting was determined from the dates of the first and last nests at each beach during each nesting season. For each beach, the number of nests per month was averaged across the nine seasons to obtain a mean temporal distribution of nesting at each site, visualized using the base 10 logarithm. The mean nest density at each site was determined by dividing the mean number of nests deposited during the seasons monitored by the length of the beach.
Because of the complex life cycle of sea turtles, in which only females come ashore to deposit their eggs, it is not possible to estimate the total population size, and therefore breeding females serve as an indicator of the status of the population in a given region (Gerrodette & Taylor, Reference Gerrodette, Taylor, Eckert, Bjorndal, Abreu-Grobois and Donnelly1999; SWOT Scientific Advisory Board, Reference Scientific Advisory Board2011). The size of the female population was estimated from total nest counts, the most easily measurable unit in a season, clutch frequency (number of nests laid by a female in a season) and the remigration interval (the interval before the same female returns to nest in the next season). The number of females nesting annually in the study area was therefore estimated by dividing the total number of nests in each nesting season by the probable clutch frequency of 1–3 nests/season (Schulz, Reference Schulz1975; Márquez, Reference Márquez1990; Kalb, Reference Kalb1999; Aguirre, Reference Aguirre2004; Metcalfe et al., Reference Metcalfe, Agamboué, Augowet, Boussamba, Cardiec and Fay2015). This clutch frequency is supported by olive ridley tracking data in Angola that show some turtles nest up to three times in a season and others nest only once (Morais & Primo, Reference Morais and Primoin press). The number of breeding females in the population was determined by multiplying the estimated number of females nesting in a season by the remigration interval of 1.5 years (Miller, Reference Miller, Lutz and Musick1997; Metcalfe et al., Reference Metcalfe, Agamboué, Augowet, Boussamba, Cardiec and Fay2015).
Results
Spatial distribution and nest density
Olive ridley nesting in Angola extended over 1,210 km from the north coast of Cabinda to the south of Ponta Albina, with no evidence of nesting found south of this latitude, coinciding with the division between the Angola and Namibe Ecoregions (Fig. 1).
The aerial surveys indicated that the northern and central coasts of Angola supported widespread olive ridley nesting. The key nesting areas were between the Xingi River and Barra do Dande, Buraco and the Longa River, Cabo das Três Pontas and the Palanca/Muconga region, Quicombo and Praia da Hanha, and Chamune and Cuio (Fig. 2).
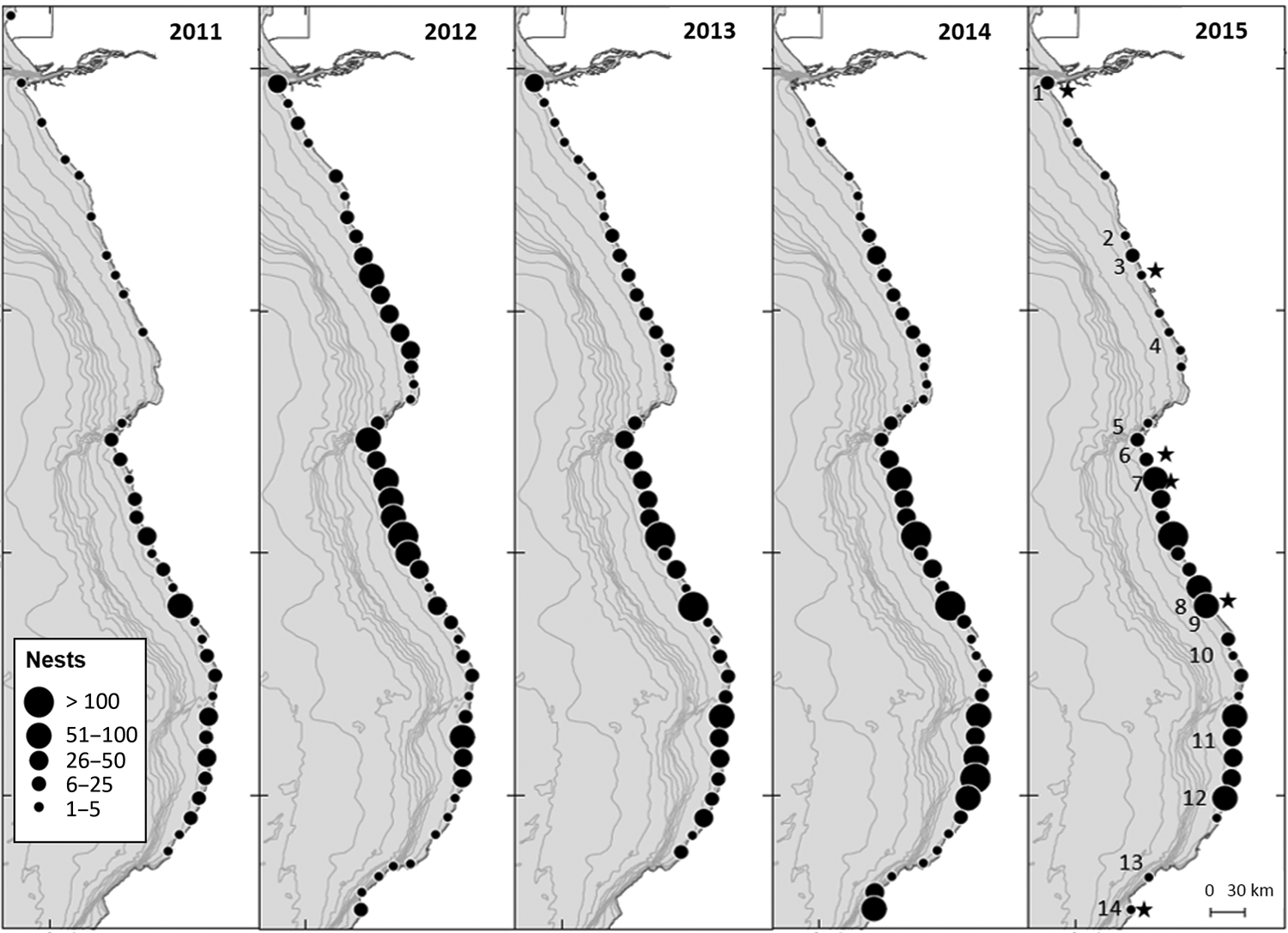
Fig. 2 Distribution of olive ridley sea turtle Lepidochelys olivacea nests along the north and central Angolan coast, identified from aerial and ground surveys (Fig. 1) during 2011–2015. Black stars, permanent monitoring sites; 1, Soyo; 2, Xing River; 3, Kissembo; 4, Barra do Dande; 5, Buraco; 6, Palmeirinhas; 7, Sangano; 8, Longa; 9, Cabo das Três Pontas; 10, Palanca Muconga; 11, Quicombo; 12, Hanha; 13, Chamume; 14, Cuio.
Of the seven permanent monitoring sites, we consistently recorded the highest number of nests each season at Longa, with an annual mean of 1,498 nests (Table 1) and a mean density of 150 nests/km. The lowest annual nesting activity was at Cuio, with a mean of 122 nests per season (Table 1) and a mean density of 21.6 nests/km. Over the 53.9 km of beaches monitored since 2014, the mean nest density was 68.6 nests/km (Table 1). Overall, nesting at the seven sites was generally stable (Fig. 3).
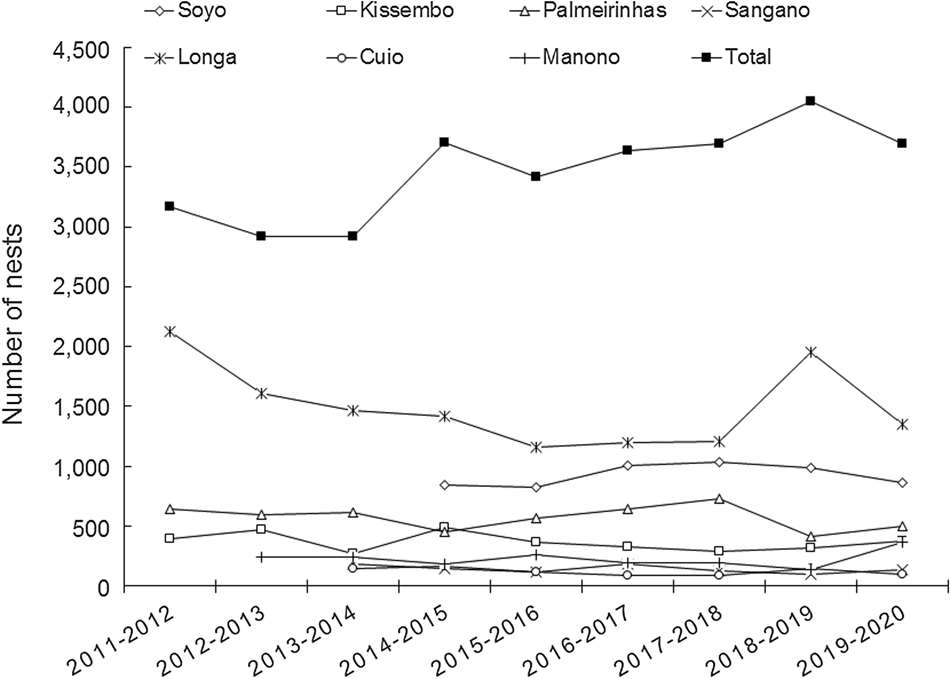
Fig. 3 Number of olive ridley nests recorded during 2011–2020 at the seven permanent monitoring sites and for all seven sites combined. Twelve-month periods are from July to June.
Seasonal nesting pattern
Olive ridley turtle nesting along the Angolan coast took place during September–April, with no nests or one nest/month during May–August (Fig. 4). At some sites nesting was low or did not occur in September or in March/April. The start of nesting varied with latitude, with nesting starting in September along the north and central coast and in October in the southern region. Peak nesting was generally during November–January (Fig. 4).
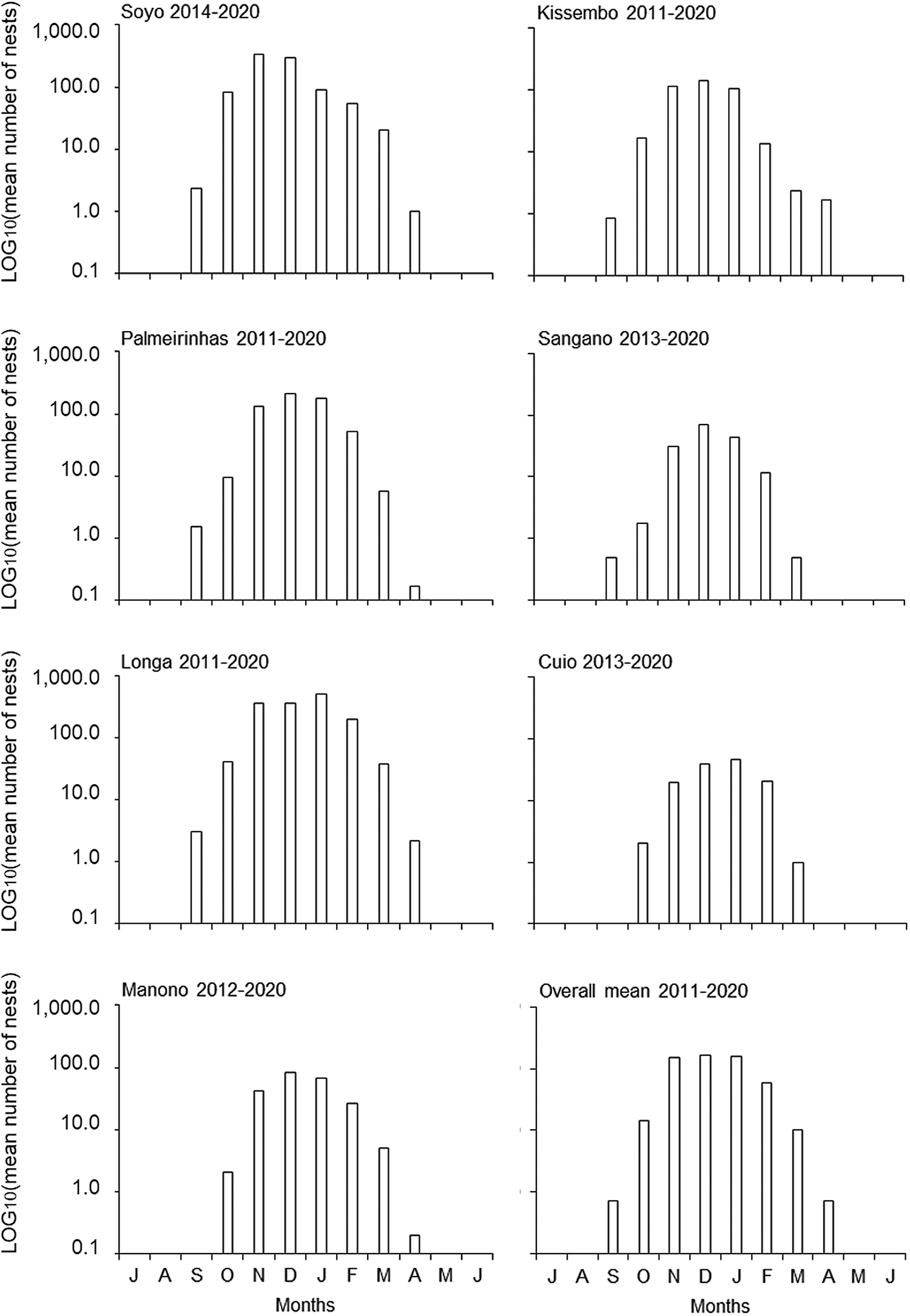
Fig. 4 Mean monthly number of olive ridley nests recorded at each of the seven permanent monitoring sites during 2011–2020. Twelve-month periods are from July to June.
Population size and trend
During 2014–2020, 3,698 nests were laid on average annually over 53.9 km of beach (range 3,419–4,050 nests/year). Using a clutch frequency of 1–3 nests/season indicates nesting by 1,233–3,698 olive ridley turtles on average each season (Table 1). The 53.9 km of beaches monitored are representative of varying nest densities at a range of latitudes along the entire Angolan coastline. Extrapolating the mean density of nests from these beaches (68.6 nests/km) to the entire 1,210 km of coastline where nesting occurs indicated that on average a total of 83,024 nests were laid annually (range 76,753–90,918 nests/year) during 2014–2020, with an estimated 27,675–83,024 females nesting on average per year (Table 1). Using the remigration interval of 1.5 years, the estimated size of the breeding population is 41,513–124,536 females. The number of nesting females, using the clutch frequency of 1–3 nests/season, across seasons at the monitored sites and along the coast of Angola appeared to be relatively stable, especially during 2014/2015–2019/2020, when 53.9 km of the coastline were consistently monitored (Fig. 5).
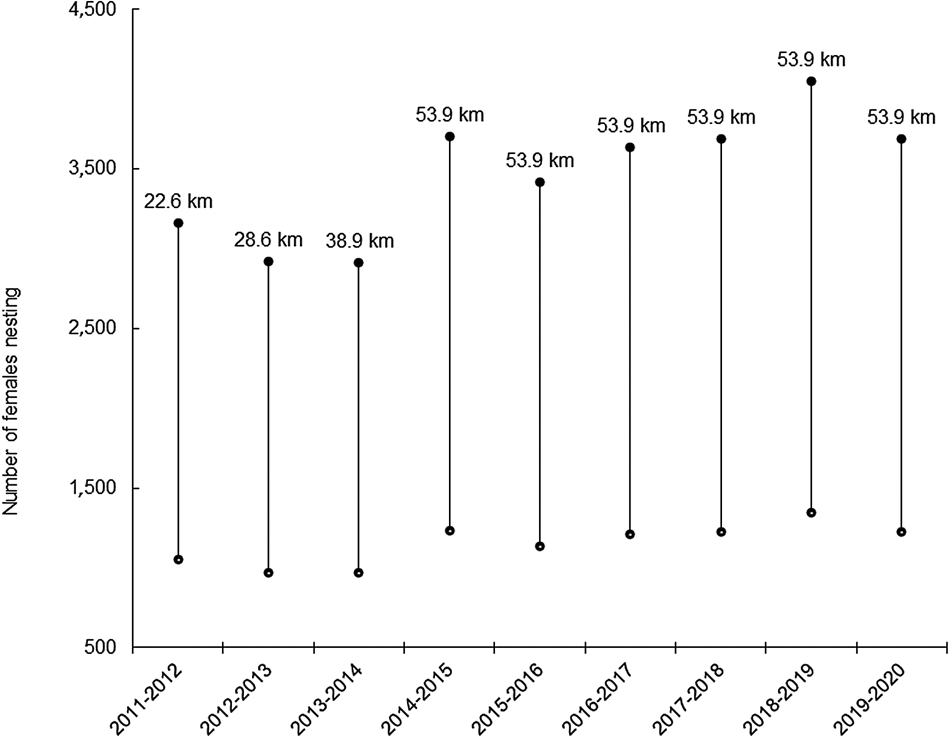
Fig. 5 Minimum and maximum number of females nesting in each season during 2011–2020 at the seven permanent monitoring sites combined, using a clutch frequency of 1–3 nests/female. Twelve-month periods are from July to June. The numbers above the bars indicate the length of beach monitored during that season.
Discussion
Our findings indicate that Angola hosts the largest nesting olive ridley turtle population in the Atlantic, without constituting an arribada, with an estimated annual mean of 83,024 nests and an estimated 27,675–83,024 females nesting. The Gabon population, previously documented as the largest breeding population for this species in the Atlantic, has an estimated median range of 2,370–9,814 clutches with 948–5,452 females nesting annually (Metcalfe et al., Reference Metcalfe, Agamboué, Augowet, Boussamba, Cardiec and Fay2015). The surveyed coastline of Angola is more than twice the length of the Gabonese coastline. Comparatively, annual nesting numbers reported from the other major olive ridley turtle nesting populations in the Atlantic are much smaller: Brazil 2,606 nests, Suriname 335 nests, French Guiana 1,000–3,300 nests and Republic of the Congo 300–600 nests (Silva et al., Reference Silva, de Castilhos, Lopez and Barata2007; Plot et al., Reference Plot, De Thoisy, Blanc, Kelle, Lavergne and Roger-Bérubet2012; Girard & Breheret, Reference Girard and Breheret2013; NMFS & USFWS, 2014). Globally, Angola appears to be the largest non-arribada population and is larger than some of the arribada populations (e.g. some arribada beaches in Mexico, Nicaragua and Costa Rica; NMFS & USFWS, 2014). Given the previous lack of long-term data for olive ridley turtles in the eastern Atlantic, this finding is important for assessments of the olive ridley turtle population at the regional and ocean-basin scales and for the global IUCN Red List assessment. This finding also reinforces the need for a comprehensive genetic assessment of olive ridley turtles in the eastern Atlantic and for an examination of the relatedness of the Angolan population to other olive ridley turtle populations (Bowen et al., Reference Bowen, Clark, Abreu-Grobois, Chaves, Reichart and Ferl1998; Ferrera et al., Reference Ferrera, Formia, Ciofi, Natali, Agyekumhene and Allman2021).
The robustness of the spatial and temporal data collected through our aerial and ground surveys of the Angolan coastline has now made it possible to identify priority nesting areas and to develop targeted conservation strategies along the coast. Although accurate population estimates of the Angolan olive ridley turtle population are hindered by the lack of country-specific or region-specific clutch frequency and remigration interval data (Metcalfe et al., Reference Metcalfe, Agamboué, Augowet, Boussamba, Cardiec and Fay2015), the estimate of clutch frequency used (1–3 nests/season) is corroborated by telemetry data of olive ridley turtles in Angola (Morais & Primo, Reference Morais and Primoin press). Estimating accurate clutch frequency data and remigration intervals for Angola through a combination of telemetry studies, consistent and intensive seasonal and spatial beach coverage, and long-term tagging of females on the beaches (Weber et al., Reference Weber, Weber, Godley, Ellick, Witt and Broderick2013; Casale & Ceriani, Reference Casale and Ceriani2020) remains a priority.
Threats to sea turtles in Angola include egg collection and the capture of turtles while they are nesting (Morais et al., Reference Morais, Andrade and Afonso2004; Buza, Reference Buza2005; Weir et al., Reference Weir, Ron, Morais and Duarte2007; Morais, Reference Morais2008, Reference Morais2011, Reference Morais2012a, Reference Morais2012b, Reference Morais2013, Reference Morais2014, Reference Morais2015, Reference Morais2016), capture in fishing gear, responsible for the deaths of thousands of sea turtles (Morais, Reference Morais2017, Reference Morais2018, Reference Morais2019), and predation of nests and hatchlings by domestic animals from communities near the nesting beaches (Morais, Reference Morais2015, Reference Morais2016). Additionally, there is high natural predation of nests by black-backed jackals Canis mesomelas in southern Angola (Morais, Reference Morais2013), and predation of adults by spotted hyaenas Crocuta crocuta in northern Angola (Morais, Reference Morais2015). Coastal dynamics of erosion and accretion of sand on the nesting beaches also result in the loss of numerous nests (Morais, Reference Morais2020).
The local coastal population is aware of the presence of sea turtles and that they are protected by law in Angola, but there is as yet no national conservation plan for sea turtles and no enforcement of the laws protecting them. Nevertheless, the olive ridley turtle population, at least at the monitored sites, has remained relatively stable. However, given the various pressures on sea turtles in Angola and the estimated mean annual capture of c. 4,000 turtles in local artisanal fishing gear (Morais, Reference Morais2020), continued protection, monitoring and minimization of threats remain necessary.
Sea turtles have been on the list of protected species in Angola since 1972, in accordance with hunting regulations at the time (Hughes, Reference Hughes and Bjorndal1982b), and in 2004 their conservation was reinforced with the Law of Aquatic Biological Resources, article No. 71, which gives them total protection. In 2006, sea turtles were identified as a priority conservation species by the Ministry of the Environment's National Biodiversity Strategy. The Angolan government signed the Memorandum of Abidjan of the Convention on Migratory Species in 2002, demonstrating its commitment to the conservation of sea turtles. However, an error occurred in the updating of the law and the new Joint Executive Decree no. 201/16 of 26 April 2016 only protects the leatherback and the loggerhead turtles, leaving the olive ridley and other sea turtle species without legal protection.
The development of a comprehensive management plan, improved and strengthened legislation and enforcement, and a cohesive approach to conserving all sea turtle species in Angola are a priority. Such actions need to be complemented by continued research on the biology, distribution and ecology and population size of all species of sea turtles in Angola.
Acknowledgements
We thank the technical and field teams of Projeto Kitabanga for data collection, and Luis Veríssimo for preparing Figs 1 and 2. We are grateful to the following sponsors and partners for their support: BP, Angola LNG, Angola Cables, Holísticos, Fundação Kissama, Ministerio Cultura, Turismo e Ambiente, Força Aérea and the U.S. Fish & Wildlife Service. We thank Peter Dutton and Jeffrey Seminoff for their reviews.
Author contributions
Study design, fieldwork: MM; data analysis, writing: MM, MT.
Conflicts of interest
None.
Ethical standards
This research abided by the Oryx guidelines on ethical standards. Data collection for the Kitabanga Project by the Faculty of Sciences of Agostinho Neto University was approved by the Ministry of Culture, Tourism and Environment of the Republic of Angola.








