Ecological studies of soil flora and fauna are widely recognized as having lagged behind those of their terrestrial counterparts. Subterranean herpetofauna (amphibians and reptiles) inhabit many areas of the globe and occur on every continent except Antarctica. In general they are elongate, have reduced or absent limbs and heads modified for burrowing. All are predators of soil macrofauna, especially earthworms, termites and ants (e.g. Webb et al., Reference Webb, Shine, Branch and Harlow2000). Until recently, knowledge of these diverse herpetofaunal groups has come from anecdotal observations in taxonomic treatise.
There are two major challenges to monitoring changes in vertebrate species below ground. Firstly, they are cryptic and difficult to sample and, secondly, little historical data exist with which to compare density estimates obtained. The first of these problems has been addressed to a limited extent by the proposal of a number of quantitative and semi-quantitative sampling techniques (Measey, Reference Measey2006). The second is more problematic as virtually no historical data exist and few baseline data are being published.
The vulnerability of subterranean vertebrates has seldom been recognized. Where known, many species appear to be well adapted to cohabitation with small-scale human agriculture (Hebrard et al., Reference Hebrard, Maloiy and Alliangana1992; Measey, Reference Measey2006) but suspected threats come from above ground processes either directly (e.g. soil compaction, which exponentially increases the force required for burrowing; Navas et al., Reference Navas, Antoniazzi, Carvalho, Chaui-Berlink, James and Jared2004), or indirectly by affecting potential food items (e.g. the impact of pesticides on abundance of soil macrofauna). The relationship between leaf-litter quantity and soil macrofauna has been well studied (Ponsard et al., Reference Ponsard, Arditi and Jost2000; Negrete-Yankelevich et al., Reference Negrete-Yankelevich, Fragoso, Newton and Heal2007). Within protected areas, changes in stocking of keystone herbivores can have profound effects on vegetation (Sinclair, Reference Sinclair2003) and, by inference, on soil macrofauna.
To our knowledge the only published study detailing historical quantitative abundance of subterranean herpetofauna was conducted in Ndumu Game Reserve, South Africa, during March and April 1970 (Pooley et al., Reference Pooley, Pooley, Hadley and Gans1973). Two habitat types were sampled within the Reserve, yielding a total of eight species and with high densities of Van Dam's round-headed worm lizard Zygaspis vandami and the lowveld dwarf burrowing skink Scelotes bidigittatus. Here we report an investigation of the current densities of subterranean herpetofauna in Ndumu Game Reserve and compare herbivore stocking levels and leaf-litter accumulation at the same two sites visited by Pooley et al. (Reference Pooley, Pooley, Hadley and Gans1973). We summarize the results of Pooley et al. (Reference Pooley, Pooley, Hadley and Gans1973) and compare them to our own, highlighting significant differences between the data sets as well as suggesting possible causal links.
Ndumu Game Reserve forms part of the north-eastern boundary of KwaZulu-Natal and therefore South Africa with Mozambique (Fig. 1). Sites visited by Pooley et al. (Reference Pooley, Pooley, Hadley and Gans1973) and resampled in this study are Ndumu Hill and uluKhondo Forest, corresponding to Pooley et al.’s (Reference Pooley, Pooley, Hadley and Gans1973) type A and B areas, respectively. The vegetation on Ndumu Hill is predominantly deciduous broad-leaf woodland on Hutton soils with grass-dominated ground cover; the vegetation of the uluKhondo Forest is classified as sand forest on Fernwood soils with a sparse understorey characterized by forbs and bushes together with numerous mounds of fungus-growing termites (Termitidae: Macrotermitinae: Odontotermes sp.; De Moor et al., Reference De Moor, Pooley, Neville and Barichievy1977; Pooley, Reference Pooley1978; AJA, pers. obs.).
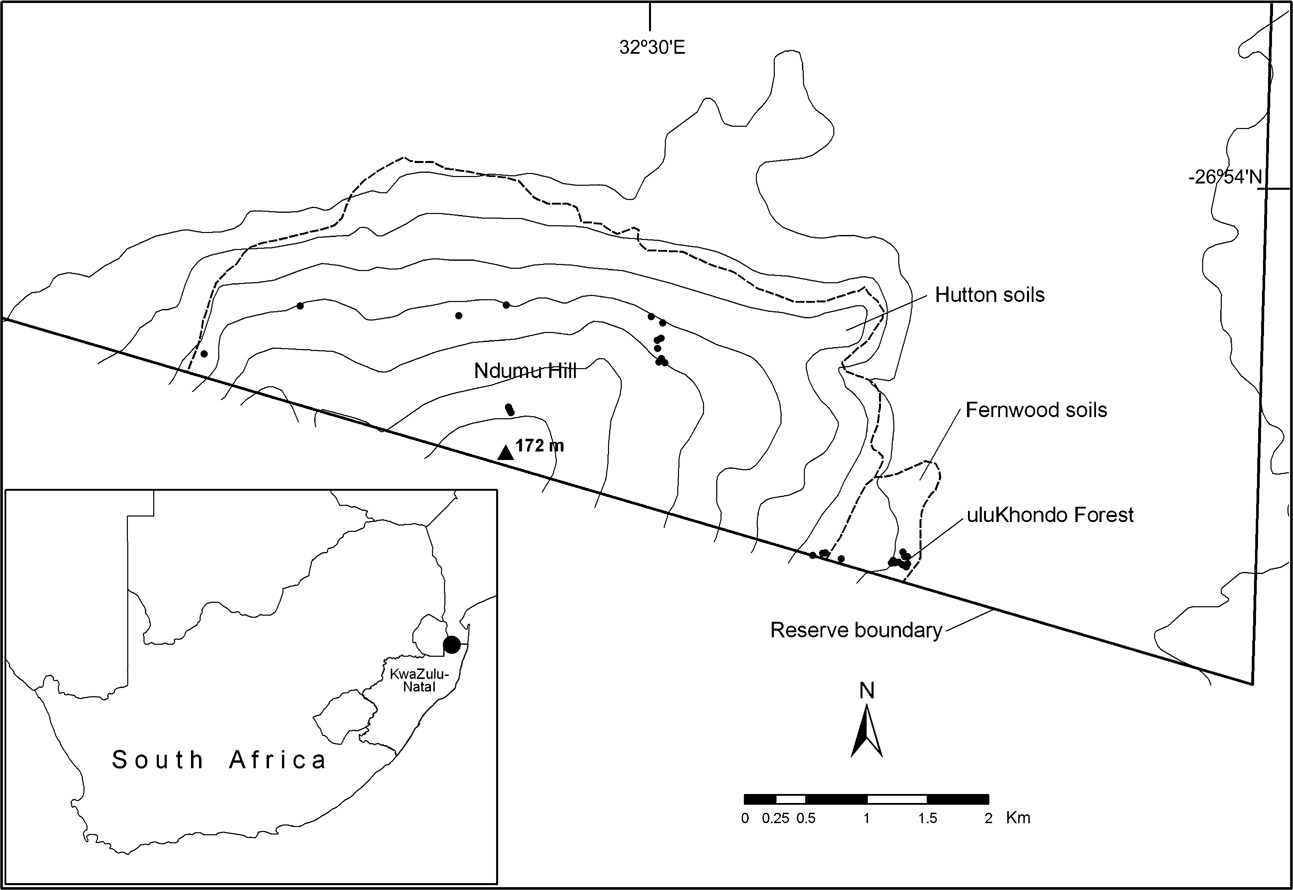
Fig. 1 Location of Ndumu Game Reserve in South Africa (filled circle on inset) and the sampling sites (dots, this study), topography and soils in the south-eastern section of the Reserve.
Pooley et al. (Reference Pooley, Pooley, Hadley and Gans1973) used various sizes of quadrats (1.67–100 m2) and sorting by hand to sample subterranean herpetofauna in March and April 1970 from named areas within Ndumu, and we reproduce their results (using metric conversions) in Table 1. We also used quadrat sampling methods, as far as possible within the original quadrats of Pooley et al. (Reference Pooley, Pooley, Hadley and Gans1973) or at most within 10 m of their quadrats, but with standardized 4 m2 quadrats, on four occasions between March 2004 and April 2005. Two observers dug during daylight hours to a depth of a hoe face (c. 0.25 m) from opposite ends of the quadrat, sorting by hand any subterranean herpetofauna encountered. Leaf litter depth was measured using a probe with the tip resting on the soil surface, and three measurements were taken per 4 m2 quadrat. All quadrats sampled in uluKhondo forest were under an estimated 40–100% tree canopy cover. On Ndumu Hill the recorded tree canopy cover over quadrats was 0–100%. The quadrats were individually placed to sample the microhabitats recorded in Pooley et al. (Reference Pooley, Pooley, Hadley and Gans1973). Identifications of all reptiles found were made with Broadley (Reference Broadley1994) and Branch (Reference Branch1998).
Table 1 Numbers (per quadrat) and densities (m-2) of two abundant species of subterranean herpetofauna in deciduous broad-leaf woodland (Ndumu Hill) and sand forest (uluKhondo Forest) at Ndumu Game Reserve, South Africa (Fig. 1). Data are from Pooley et al. (Reference Pooley, Pooley, Hadley and Gans1973), collected in 1970 and converted to metric, and from this study (2004–2005). Total shows overall numbers and density (total number divided by area dug), and the mean and standard deviation are calculated from each quadrat sampled as the number of animals (No.) and in proportion to the area sampled (Density).
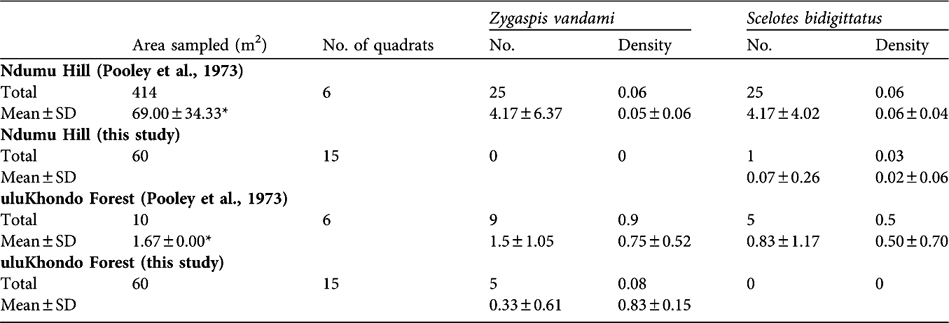
* Pooley et al. (Reference Pooley, Pooley, Hadley and Gans1973) used different sizes of quadrats and here we present their mean and standard deviation.
To compare the two studies we calculated the density (m-2) of both lizard species within each quadrat independent of sampling occasion. Mann-Whitney tests were then performed on these data in SPSS v. 9.0 (SPSS, Chicago, USA) using exact significance (two times the one-tailed significance). Pooley et al. (Reference Pooley, Pooley, Hadley and Gans1973) found relatively high differences in subterranean herpetofaunal densities between the two habitat types, with Ndumu Hill having much higher densities of Z. vandami and S. bidigittatus than uluKhondo Forest (Table 1). Our data indicate lower densities of Z. vandami and S. bidigittatus in both habitats (Table 1). We found only one of the other six species of subterranean herpetofauna listed by Pooley et al. (Reference Pooley, Pooley, Hadley and Gans1973): two specimens of the Zululand dwarf burrowing skink Scelotes arenicolus were unearthed in quadrats on Ndumu Hill, whereas Pooley et al. (Reference Pooley, Pooley, Hadley and Gans1973) found this species only in uluKhondo Forest.
Comparing data from Pooley et al. (Reference Pooley, Pooley, Hadley and Gans1973) and data collected in this study, a decline in overall abundances of subterranean herpetofauna appears to be most marked for Z. vandami in both deciduous broad-leaf woodland (Mann-Whitney U = 7.50, P = 0.002) and sand forest (Mann-Whitney U = 9.50, P = 0.003). This subspecies (Z. v. arenicola) has a limited distribution on the coastal sands of northern South Africa and south-eastern Mozambique (Branch, Reference Branch1998). While there is little life-history information published, a detailed study of a congener, Z. quadrifrons, indicates they eat mostly termites and other soil macrofauna, and that they have a relatively small mean clutch size of 3.3 eggs (Webb et al., Reference Webb, Shine, Branch and Harlow2000). Given its vulnerability to changes in land use and limited distribution, it is likely that Z. v. arenicola needs to be categorized in an IUCN threatened category (IUCN, 2001).
We found that S. bidigittatus densities were significantly lower at Ndumu Hill (Mann-Whitney U = 6.00, P = 0.001) but non-significantly so in the sand forest (Mann-Whitney U = 22.50, P = 0.080). S. bidigittatus has two small hind limbs each bearing two toes, inhabits the litter and humus layers of the soil, and so may be affected by changes in leaf-litter characteristics (Branch, Reference Branch1998).
Pooley et al. (Reference Pooley, Pooley, Hadley and Gans1973) recorded plant litter depths of up to 300 mm on Ndumu Hill, whereas we recorded mean litter depths in April 2005 of 14.1 ± SD 6.7 mm (n = 18) on Ndumu Hill and 14.4 ± SD 4.9 mm (n = 18) in uluKhondo Forest. A reason for the decrease in litter depths may be the marked increase in the number of certain ungulates. Impala Aepyceros melampus reached a population size of 500 by the end of 1965 and nyala Tragelaphus angasii had reached 2,500 by 1972. These antelope populations continued to increase and in 2000 they were 2,992 and 5,302 impala and nyala, respectively. Nyala are predominantly grazers during the rains in the Reserve, whereas their diet during drier months is dominated by leaves (including fallen leaves) and fruits of dicotyledons (Van Rooyen, Reference Van Rooyen1992). Impala are also grazers, and the proportion of dicotyledons in their diet undergoes similar increases in the dry season (Van Rooyen, Reference Van Rooyen1992). The high number of impala and nyala in the Reserve appears to have had a detrimental effect on some of the vegetation, changing habitat structure and decreasing food available to other ungulates (Pooley, Reference Pooley1978; Adcock, Reference Adcock2004). We know of no other factors that may be responsible for this reduction in leaf litter. While the link between wild ungulate stocks, leaf litter and subterranean vertebrates is not proven by this study, we believe that the known link between soil macrofauna and leaf-litter levels, and the commensurate reliance by subterranean vertebrate predators on these macroinvertebrates is more than suggestive of the association. More directly, the increase in ungulate densities may have had a direct effect on amphisbaenians through soil compaction, reducing their dispersal abilities (Navas et al., Reference Navas, Antoniazzi, Carvalho, Chaui-Berlink, James and Jared2004).
Although little studied, subterranean herpetofauna may represent as much as 20% (nearly 3,000 species) of the world's reptiles and amphibians (Measey, Reference Measey2006). The conservation status and life history of many of these species is unknown. The results of this study suggest that some subterranean reptiles are negatively affected by high densities of ungulates. This should be taken into account by reserve managers when planning stock densities, and may also be relevant for determining the reptiles' conservation status using IUCN (2001) criteria. This study also emphasizes the need for quantitative baseline data on subterranean herpetofauna to monitor densities.
Acknowledgements
We acknowledge the help of John Craigie and Robert Karssing for assistance in the field, and two anonymous reviewers for comments on an earlier draft. Thanks also to Heidi Snyman for her help with the figure.
Biographical sketches
John Measey has studied subterranean herpetofauna for more than 10 years, developing standardized sampling methodologies and obtaining life history data for many African species. Adrian J. Armstrong works on the conservation of endemic and threatened invertebrates and herpetofauna in KwaZulu-Natal, including the collection and collation of distribution records, species distribution modelling, target-setting for species conservation, reintroductions and monitoring. Cathariné Hanekom has worked on the determination of game stocking levels, appropriate game management interventions and uses, and vegetation carrying capacities for EKZN Wildlife and community conservation areas. More recently she has become involved in the compilation of sustainable resource utilization management plans for protected areas, particularly for forest reserves.




