Introduction
The Egyptian vulture Neophron percnopterus is a territorial, cliff-nesting raptor distributed from the Mediterranean countries to India and also occupying areas in the east and south of Africa. Formerly categorized as Least Concern on the IUCN Red List the species has recently been upgraded to Endangered (BirdLife International, 2008a) because of its rapid population decline in India (up to 35% annually during 2000–2003; Cuthbert et al., Reference Cuthbert, Green, Ranade, Saràvanan, Pain, Prakash and Cunningham2006) and Europe (> 50% over the last three generations; BirdLife International, 2008a).
In Europe, where there are 3,300–5,050 breeding pairs, the Egyptian vulture is also categorized as Endangered (BirdLife International, 2004). The threats to the species are poisoning, human persecution and disturbance, loss of suitable habitat, collisions with power lines and wind farms, and reduced food availability (BirdLife International, 2008b). In Spain, which contains 82% of the European population, the species is categorized as Endangered because of a population decline of c. 25% detected over 1987–2000 (Donázar, Reference Donázar, Madroño, González and Atienza2004). However, the species exhibits differing population trends in different parts of the country. In some areas declines of up to 60% have been described during the last 2 decades (in the Ebro valley, Navarra: Del Moral & Martí, Reference Del Moral and Martí2002; Cortés-Avizanda et al., Reference Cortés-Avizanda, Ceballos and Donázar2009), whereas population increases have been reported in other regions over the same period (COA, 2004; García-Ripollés & López-López, Reference García-Ripollés and López-López2006). Some of the increases could be an artefact of improved monitoring techniques (Donázar, Reference Donázar, Madroño, González and Atienza2004) but could also be because of the presence of extensive livestock activity in some areas (Donázar, Reference Donázar, Madroño, González and Atienza2004), which has been shown to be crucial for maintaining the species’ territories in mountainous areas (Mateo-Tomás & Olea, Reference Mateo-Tomás and Olea2010). The populations that show stable or increasing trends may be key for the species’ conservation within a metapopulation context.
Here we examine the status, population trends and breeding parameters of the Egyptian vulture population in the western and central Cantabrian Mountains. This region accounts for c. 20% of the total Spanish population (Del Moral & Martí, Reference Del Moral and Martí2002). We also assess the main threats to the species (poisoning, human disturbance, reduced food availability and wind farms; BirdLife International, 2008b) in this Cantabrian population.
Study area
The Egyptian vulture is widespread in the Iberian Peninsula but it is absent from areas that are topographically uniform or that have a dry climate. We studied Egyptian vultures in four regions in north-west Spain: Asturias, western Cantabria, León and Palencia (Fig. 1). This area extends over 15,500 km2. The central part of the studied area is characterized by the presence of high, rocky mountains (up to 2,648 m) and the western portion contains both high, rocky mountains and areas of lower altitude, mainly dominated by forests. The Egyptian vultures in this area are at the north-western edge of their distribution in Spain and Europe (Fig. 1). Breeding pairs arrive from their winter range in Africa during early March and remain in their breeding territories until mid September, rearing one or two chicks. A territory is generally used year after year and is actively defended by its occupants (Donázar, Reference Donázar and Reyero1993).
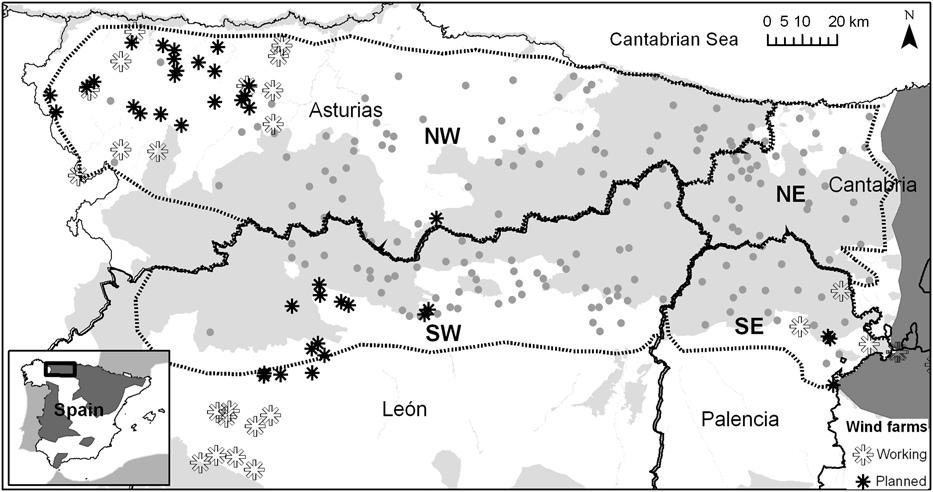
Fig. 1 The study area in the Cantabrian Mountains of north-west Spain. The grey dots are Egyptian vulture Neophron percnopterus territories. The solid black lines represents administrative boundaries and the dashed lines the limits of the NW, NE, SW and SE sectors (both lines overlap partially). Protected areas are shown in grey. Locations of the wind farms currently working and those approved for construction within the next few years are also shown. The dark shaded area on the inset shows the species’ breeding range in Spain (Del Moral & Martí, Reference Del Moral and Martí2002) and the rectangle the location of the main figure.
Methods
Data on the historical distribution of the Egyptian vulture population in the study area were gathered from previous censuses (Perea et al., Reference Perea, Morales and Velasco1990; Benito et al., Reference Benito, González-Quirós and Del Campo1997; Jubete, Reference Jubete1997; Del Moral & Martí, Reference Del Moral and Martí2002; COA, 2004; Picos de Europa National Park, pers. comm.; authors, unpubl. data). Up-to-date data were obtained from Del Moral (Reference Del Moral2009) and from field surveys in 2008 (Fombellida et al., Reference Fombellida, Gómez and Saiz2008). The censuses consisted of visiting areas that could be suitable for the species (i.e. areas with rocky cliffs) between March and August. Visits included inspection of the cliffs using binoculars and telescopes on days with good visibility. The mean number of visits per territory varied between the different censuses, from a mean of 3 ± SE 0 to 6 ± SE 2). The Egyptian vulture breeding territories were classified according to the amount of evidence for the occurrence of breeding following the terminology proposed by Martí & Del Moral (Reference Martí and Del Moral2003).
Data analysis
The study area was divided into four sectors according to regional boundaries because most available data were obtained from regional censuses (Fig. 1, Table 1). These sectors are: (1) NW, Asturias province, monitored in 1988, 1991, 1996, 2000, 2004 and 2008, (2) NE, western Cantabria province, monitored in 1988, 2000 and 2008, (3) SW, León province, monitored in 1988, 2000 and 2008, and (4) SE, Palencia province, monitored in 1988, 1997, 2000 and 2008.
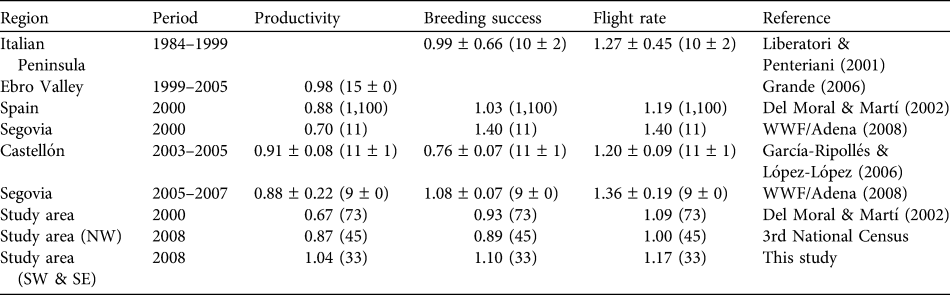
Table 1 Breeding parameters (mean ± SD) of Egyptian vulture Neophron percnopterus populations in the Italian Peninsula and in various regions of Spain. Sample sizes (equivalent to mean number of monitored pairs per year ± SD) are given in parentheses.
The population trend was calculated as the variation (%) in the number of breeding pairs between different time periods (Del Moral & Martí, Reference Del Moral and Martí2002). In the SW and SE sectors of the area, where the censuses were sufficient (mean = 6 visits per territory per year; n = 394) to identify territory occupation with a high probability (95%; authors, unpubl. data), we estimated the percentage of new (or not previously detected) and abandoned territories for 2000–2008. Accordingly, we considered a territory abandoned if it had been occupied previously but if in 2008 we did not detect Egyptian vultures within the home range (2.5 km; Mateo-Tomás & Olea, Reference Mateo-Tomás and Olea2009, Reference Mateo-Tomás and Olea2010).
Only those pairs that were adequately monitored throughout the breeding season were used to calculate the breeding parameters: productivity (number of fledglings/number of monitored pairs), breeding success (number of fledglings/number of incubating pairs) and flight rate (number of fledglings/number of pairs with fledglings; Del Moral & Martí, Reference Del Moral and Martí2002). When the census methodologies were similar, we compared breeding parameters using Kruskal-Wallis and Wilcoxon signed rank tests.
We evaluated the threats that are considered critical or important according to the Species Action Plan for the Egyptian vulture in the European Union (BirdLife International, 2008b). Poisoning data for 2000–2008 were obtained from the Antidoto Program database (WWF-ADENA, 2008). The total number of poisoning events was computed for the Egyptian vulture and for other scavenging birds (including griffon vulture Gyps fulvus, kites Milvus sp. and corvids), non-scavenging birds and mammals.
Reduced food availability is considered a critical threat for the species (BirdLife International, 2008b), and extensive livestock (i.e. free-ranging cows, sheep and goats at low stocking densities) is key for the presence of Egyptian vulture territories in the study area (Mateo-Tomás & Olea, Reference Mateo-Tomás and Olea2009, Reference Mateo-Tomás and Olea2010). We used data from regional and national government databases to calculate the current density of livestock units (LU) in the study area (1 cow/horse = 5 LU, 1 sheep/goat = 1 LU; Olea & Mateo-Tomás, Reference Olea and Mateo-Tomás2009) per municipality (LU km-2) and any trends during the last decade. To collect data on the removal of livestock carcasses we gave questionnaires to local and transhumant shepherds (n = 97) and to five veterinary units in 2005–2008. We interviewed 85 shepherds (55% of those in the area) and 12 cowherds (2% of those in the area). We obtained information about the removal of livestock carcasses for > 50% of the cow breeders (n = 625) through the official databases of the veterinary units.
Collision with wind turbines is an emerging threat to Egyptian vultures (BirdLife International, 2008b; Carrete et al., Reference Carrete, Sánchez-Zapata, Benítez, Lobón and Donázar2009). We calculated the number of wind turbines that have been established in the study area and the number that will become established over the next few years. To assess the potential impact of these structures on the conservation of the studied population we calculated the number of breeding territories that have wind turbines located within a radius of 25 km (reported as the maximum foraging distance from the nest for Egyptian vultures; Bergier & Cheylan, Reference Bergier and Cheylan1980).
Disturbance by human activities and habitat degradation are also threatening the Egyptian vulture (Carrete et al., Reference Carrete, Grande, Tella, Sanchez-Zapata, Donázar, Diaz-Delgado and Romo2007; Zuberogoitia et al., Reference Zuberogoitia, Zabala, Martínez and Azcona2008). To analyse the possible impact of these threats on the conservation of the species we calculated the percentage of breeding territories within and outside Natura 2000 protected areas (Sites of Community Importance and Special Protection Areas).
Results
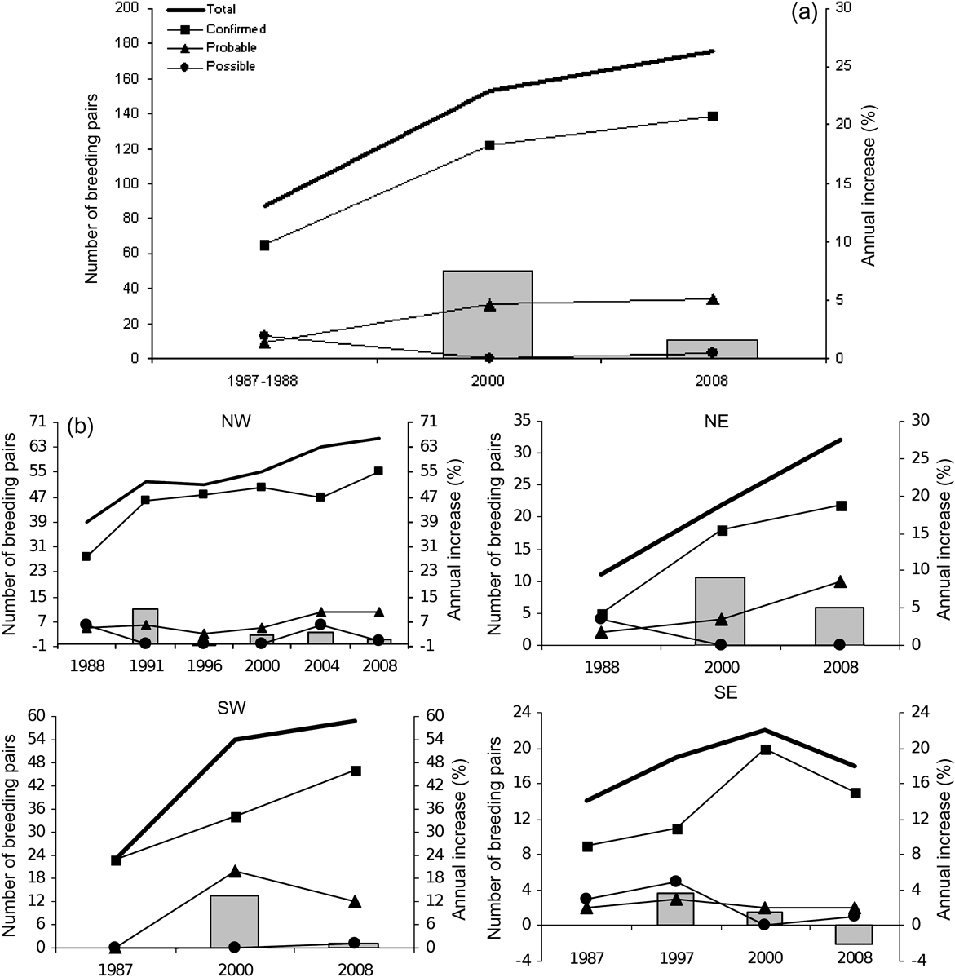
An estimated 87 Egyptian vulture breeding pairs were in the study area in 1987–1988, 153 in 2000 and 175 in 2008 (Fig. 2a,b). In three of the four sectors (NW, NE and SW) the population grew slightly in the last decade, with annual increases of 1–2% (Fig. 2b). In contrast, the population trend in the SE sector showed a decrease of 1–2% annually in the last decade (Fig. 2b).
Between 2000 and 2008 seven territories were abandoned in the SW sector (13.0% of the territories in 2000) and two territories were abandoned in the SE (9.1%). Seventeen breeding territories not detected in the 2000 census were registered as occupied in the SW and SE sectors in 2008 (22.1% of the territories located in the SW and SE sectors). Sixteen of these new breeding territories were located in the SW sector and only one in the SE sector.
The breeding parameters calculated in 2000 did not differ significantly between those regions with available data (NW, NE and SW; Kruskal-Wallis test, P > 0.05). In addition, no significant differences were found in the breeding parameters between the SW and SE sectors in 2008 (Wilcoxon test, P > 0.05). We could only compare the breeding parameters between different time periods in the NW sector, where the census methods were similar over the last 2 decades. No significant differences were found (Wilcoxon test, P > 0.05). The breeding parameters of the Egyptian vulture in the study area are similar to those reported for other regions (Table 1).
Conservation threats
One hundred poisoning events were reported in the study area during 2000–2008. These affected 201 individuals of 20 different species, including 15 Egyptian vultures (7.5%; Fig. 3). Scavenging birds were the most affected guild, with 53.7% of the dead individuals belonging to this group (Fig. 3). The SE sector accounted for 43.1% of the dead animals and 35 poisoning events (0.021 km-2). This sector had also the highest number of poisoned Egyptian vultures (six) and 49.1% of the poisoned scavenging birds. The sector with the fewest reported poisoning events was the NE (0.003 km-2, n = 5).
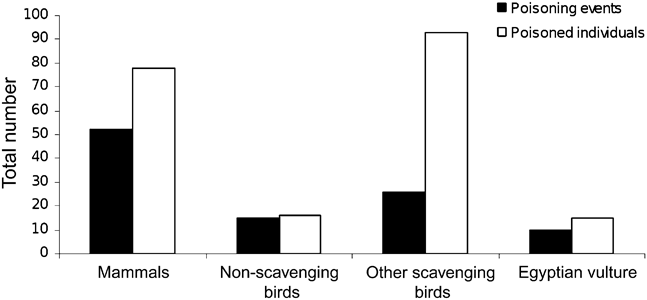
Fig. 3 Numbers of poisoning events and poisoned individuals of Egyptian vultures and other species in the study area (Fig. 1).
There are currently 19 working wind farms with 490 turbines in the study area (Fig. 1) and 32 Egyptian vulture territories (18.3% of the population) contain wind turbines within their foraging areas (mean 59 ± SE 8; range 1–176). There are an additional 40 wind farms that have been approved (1,138 turbines; Fig. 1), which will increase the number of territories that overlap with wind turbines by 116% (i.e. 69 territories in total). Three new wind farms will be located in areas densely populated with Egyptian vultures (Fig. 1). The mean number of turbines per affected territory will increase by 147%, to 110 ± SE 16 turbines per territory (range 1–707). This increase will be most noticeable in the SW sector (61% more turbines per territory) and will be smaller in the SE and NE sectors (4–13%).
The mean number of livestock units per municipality in the study area in 2008 was 123 ± SE 13 km-2, which represents a decline in livestock density of 13.6% in the last 10 years. The decrease has been greater for sheep and goats (27.4%) than for cows (6.9%). However, there are different trends between sectors. The number of cows increased by 15.2% in the last decade in the NW and SW sectors, particularly in the SW sector (134% increase). In contrast, the number of sheep and goats decreased in all sectors by 21–36%. Interviews with shepherds and veterinary authorities indicated that while only 20% of sheep and goat carcasses are removed from the field, 50% of cow carcasses are removed.
A total of 118 (67.4%) of the 175 Egyptian vulture territories in the study area were inside Natura 2000 protected areas. More than half (56.6%) of the breeding territories were within Special Protection Areas, protected sites classified in accordance with the EU Wild Birds Directive (79/409/EEC). The SE sector had a significantly greater number of Egyptian vulture breeding territories within Special Protection Areas (83.3%; Fisher’s exact test, P < 0.05 for all comparisons).
Discussion
Population status
The estimated Egyptian vulture population in the study area was 175 breeding pairs in 2008, which equates to 13–14% of the Spanish population (BirdLife International, 2008b). This represents a slight annual increase (1.6%) over the previous census in 2000 (Fig. 2; Del Moral & Martí, Reference Del Moral and Martí2002). However, this increase could be an artefact of better censusing techniques (Donázar, Reference Donázar, Madroño, González and Atienza2004), and the high annual population increase (7.6%) detected during 1987–2000 was probably due to incomplete monitoring in 1987–1988 (Del Moral & Martí, Reference Del Moral and Martí2002). Moreover, up to 25% of occupied territories could remain undetected in the study area when performing the three visits per territory recommended by the Egyptian Vulture National Census (authors, unpubl. data). During the intensive census that we carried out on the south slope of the study area, for example, we detected 17 new territories that could have been undetected in the previous census, representing 22% of the territories located in 2000.
In the SW sector the population increased by 0.15% annually during the last decade. Although this increase could be due in part to previous detection errors (see above), a slight increase was also observed during the last decade in the NW sector (Fig. 2) where good coverage (four visits per territory) and high census frequency (Fig. 2b) provide high quality data. Therefore, the inferred trend for the studied population as a whole could be considered stable or slightly increasing. Nonetheless, it is necessary to implement efficient monitoring, with good coverage and frequency of censuses, to determine the real trend of this important population.
Conservation threats
Poisoning has been highlighted as one of the main threats to the Egyptian vulture in Europe (BirdLife International, 2008b), and Spain is no exception (Hernández & Margalida, Reference Hernández and Margalida2009). In our study area 15 Egyptian vultures were found poisoned in 2000–2008 (Fig. 3), representing 4.3% of the current breeding population. This percentage is considerably lower than those reported in other areas for the same period (e.g. 45.5% in Hoces del Riaza and 30.4% in Bárdenas Reales; WWF/Adena, 2008; Cortés-Avizanda et al., Reference Cortés-Avizanda, Ceballos and Donázar2009). The density of poisoned Egyptian vultures registered in the study area in the last decade (0.0001 year-1 km-2) was considerably lower (64–140 times) than densities reported in other regions (e.g. 0.0140 year-1 km-2 in Hoces del Riaza and 0.0064 year-1 km-2 in Bárdenas Reales; WWF/Adena, 2008, Cortés-Avizanda et al., Reference Cortés-Avizanda, Ceballos and Donázar2009). Although discrepancies in the detection of poisoning events between areas may potentially account for these differences, the low figures in our study area could be also related to the lack of small hunting preserves; illegal control of predators on such properties has been highlighted as the main cause of Egyptian vulture poisoning in Spain (Hernández & Margalida, Reference Hernández and Margalida2009). Poisoning events have not been highlighted as a significant factor in the abandonment of Egyptian vulture territories in the study area (Mateo-Tomás & Olea, Reference Mateo-Tomás and Olea2010), in contrast to their apparent impact at a national scale (Carrete et al., Reference Carrete, Grande, Tella, Sanchez-Zapata, Donázar, Diaz-Delgado and Romo2007). Nonetheless, despite having relatively low rates of poisoning compared to other parts of Spain, the use of poison needs to be controlled, particularly in the SE sector, which had the highest number of poisoned Egyptian vultures and a negative population trend, to guarantee the long-term viability of the population.
The decline in food availability is another critical threat to many European Egyptian vulture populations (BirdLife International, 2008b). The extensive system of livestock rearing, together with the complex topography of our study area, make it difficult to collect carcasses from the field, a requirement of new European sanitary regulations (Olea & Mateo-Tomás, Reference Olea and Mateo-Tomás2009). Therefore, although these regulations are likely to affect scavengers (Hartasánchez et al., Reference Hartasánchez, Pando, Purroy and Magadán2006), the situation in our study area may not be as serious as that reported in other areas with intensive farming activity (Camiña, Reference Camiña2007). Moreover, the presence of the carcasses of freely roaming livestock could reduce the high dependence that the species has on muladares (i.e. supplementary feeding stations) in other regions (Carrete et al., Reference Carrete, Grande, Tella, Sanchez-Zapata, Donázar, Diaz-Delgado and Romo2007). Feeding on carcasses from freely roaming livestock also minimizes the risk to vultures of intoxication and disease resulting from veterinary drug ingestion (Blanco et al., Reference Blanco, Lemus, Martínez, Arroyo, García-Montijano and Grande2009). However, food shortages could affect scavengers in the study area in the near future because of the abandonment of extensive livestock production (especially of sheep and goats). This traditional form of production is believed to be the main factor favouring the persistence of Egyptian vultures in the study area (Mateo-Tomás & Olea, Reference Mateo-Tomás and Olea2010).
In Europe the rapid development of wind farms is threatening soaring birds, including the Egyptian vulture (BirdLife International, 2008b). In Spain it has been suggested that these structures may have a serious negative impact on the population viability of Egyptian vultures (Carrete et al., Reference Carrete, Sánchez-Zapata, Benítez, Lobón and Donázar2009). Although no Egyptian vultures have been reported as dying from collision with wind turbines in the study area (Fondo para la Protección de los Animales Salvajes and Plataforma para la Defensa de la Cordillera Cantábrica, pers. comms) the number of affected territories and the number of turbines per territory are likely to increase greatly in the next few years. Sixty-nine (39%) of the territories in the study area will overlap with wind turbines in the near future, with some territories containing several hundred turbines. The strong negative impact of wind farms on the species (Carrete et al., Reference Carrete, Sánchez-Zapata, Benítez, Lobón and Donázar2009) means that collision with wind turbines could become an important threat for this population. There is therefore a need for effective surveillance and control measures, such as those implemented in other regions (Carrete et al., Reference Carrete, Sánchez-Zapata, Benítez, Lobón and Donázar2009).
The high percentage (65.7%) of Egyptian vulture territories located within Natura 2000 protected areas could facilitate the conservation of the studied population through better surveillance and control of those activities potentially affecting the species (i.e. poisoning, human disturbance, habitat degradation and wind farms; Carrete et al., Reference Carrete, Grande, Tella, Sanchez-Zapata, Donázar, Diaz-Delgado and Romo2007, Reference Carrete, Sánchez-Zapata, Benítez, Lobón and Donázar2009; Zuberogoitia et al., Reference Zuberogoitia, Zabala, Martínez and Azcona2008). However, any decrease in food resources (as a consequence of livestock decline, for example) could force Egyptian vultures to forage over larger areas, increasing the impact of threats such as poisoning and collision with wind farms (Cortés-Avizanda et al., Reference Cortés-Avizanda, Ceballos and Donázar2009).
The relatively low impact of conservation threats such as poisoning, food shortages or collision with wind farms could explain the relatively high quality of Egyptian vulture territories in the Cantabrian Mountains (Grande et al., Reference Grande, Serrano, Tavecchia, Carrete, Ceballos and Díaz-Delgado2009). Currently the Egyptian vulture population of the NW sector is the only one in the Iberian Peninsula with an official Recovery Action Plan (Decreto 135/2001; BOPA, 2001). This Plan could be a useful tool for implementing actions that will facilitate the conservation of the species. However, this Plan affects only 38% of the studied breeding population. Implementation of conservation actions and plans and cooperation between the regional administrations are therefore needed to guarantee the conservation of this important Cantabrian population of Egyptian vultures.
Acknowledgements
We thank J.C. del Moral for access to the 2nd and 3rd Spanish surveys of Egyptian vultures and F. Jubete and Parque Nacional de Picos de Europa who provided information about nesting places and breeding parameters. Fondo para la Protección de los Animales Salvajes and Plataforma para la Defensa de la Cordillera Cantábrica provided data on wind farms. J. Tomás, J. and J.A. Herrero, J. Saiz, J. Gómez, M. Gordaliza, S. Bayón, L. Díaz and F. Carcedo provided field assistance. X. Martín, E. Gómez and the Environmental Agents of Junta de Castilla y León in Palencia participated in the census in Palencia, and J. Fernández, Uda, Iñaki and M.A. López collaborated in the census in León during 2008. We thank all the farmers who kindly answered our questions and the Veterinary Units of Junta de Castilla y León. PM-T was supported by a PhD scholarship from the Spanish Ministerio de Educación y Ciencia. IE University partially funded this study.
Biographical sketches
Patricia Mateo-Tomás researches the conservation and management of vultures and she is currently studying the impacts of traditional human activities on the conservation of biodiversity. Pedro P. Olea carries out conservation biology research with a particular interest in birds. He is currently leading a multidisciplinary team focused on biodiversity conservation, analysing the impact of human activities on ecosystem conservation. Isidoro Fombellida is an environmental consultant.