Introduction
Parrots play a significant role in conservation as their charisma and aesthetic appeal make them ideal flagship species (Snyder et al., Reference Snyder, McGowan, Gilardi and Grajal2000). Their conservation, however, remains a significant challenge. Almost 30% of the c. 330 parrot species are threatened (compared to 13% of all birds, Hoffmann et al., Reference Hoffmann, Hilton-Taylor, Angulo, Böhm, Brooks and Butchart2010), primarily because of habitat destruction or capture for the pet trade (Snyder et al., Reference Snyder, McGowan, Gilardi and Grajal2000), and the Psittacidae is the avian family with the highest number (both relative and absolute) of taxa on the IUCN Red List (Collar, Reference Collar2000). Twelve parrot species are known to have become extinct since 1600 and historical evidence suggests that others have also been lost (Stattersfield, Reference Stattersfield and Mountford1988). Ten of these documented extinctions were endemic island species. Such species have typically restricted ranges and population sizes, which make them particularly vulnerable to the effects of habitat loss, human persecution and the introduction of exotic competitors, predators or diseases. Assessing threats and conducting research into range, population size and densities are therefore essential for conservation management (Collar, Reference Collar2000).
The Seychelles black parrot Coracopsis (nigra) barklyi has one of the most restricted distributions of the Seychelles’ endemic land birds, with a current and likely historical range including only the islands of Praslin and Curieuse (Evans, Reference Evans1979; Watson, Reference Watson and Stoddart1984; Fig. 1a). The Coracopsis genus, endemic to the islands of the Indian Ocean, was formerly considered to contain two species: the greater and lesser vasa parrots (C. vasa and C. nigra, respectively). Both species are categorized as Least Concern on the IUCN Red List (IUCN, 2011) because they occupy a large range, including Madagascar and the Comores (C. vasa), with C. nigra additionally occurring on Mayotte. The Seychelles black parrot C. (n.) barklyi has formerly been regarded as a subspecies of C. nigra although it exhibits differences in size, plumage and behaviour to other C. nigra subspecies (Rocamora & Skerrett, Reference Rocamora, Skerrett, Fishpool and Evans2001; Asmus, Reference Asmus2005). Recent phylogenetic research has shown that the Seychelles population has a long history of isolation and was the first of the C. nigra group to diverge (Kundu et al., Reference Kundu, Jones, Prys-Jones and Groombridge2011). Determining the size and distribution of this small island population is therefore a priority as the current IUCN categorization may not adequately reflect the conservation status of this population.

Fig. 1 The islands of Curieuse (2.87 km2) and Praslin (38 km2), with (a) altitude contours shaded in 50-m bands, and (b) with habitat type (see text for details). The inset indicates the location of the Seychelles archipelago in the Indian Ocean.
The centre of the distribution of C. (n.) barklyi is the mature palm forest of Praslin (a forest dominated by several of the six palm species endemic to the Seychelles), with the highest densities in the UNESCO World Heritage site of the Vallée de Mai (Gaymer et al., Reference Gaymer, Blackman, Dawson, Penny and Penny1969; Evans, Reference Evans1979; Walford, Reference Walford2008). The parrot is a flagship species for Praslin, and particularly the Vallée de Mai, as the endemic palm species are a main component of the parrot's diet and the high density of coco de mer Lodoicea maldivica provides ideal nesting habitat. The island of Curieuse is also commonly reported to be part of the range of C. (n.) barklyi but documented sightings are rare (e.g. Hill et al., Reference Hill, Vel, Parr and Shah2002).
There have been several attempts to estimate the minimum population size and population trends of C. (n.) barklyi. Gaymer et al. (Reference Gaymer, Blackman, Dawson, Penny and Penny1969) reported the population to be almost exclusively located in and around the Vallée de Mai and estimated it at no more than 50 individuals. Evans (Reference Evans1979) estimated a total of 90 (± 20) individuals on Praslin using simultaneous counts, with 65% counted in the palm forest region of the Vallée de Mai. Both assessments were considered to be underestimates by Watson (Reference Watson and Stoddart1984) because they neglected areas outside the Vallée de Mai. Merritt et al. (Reference Merritt, Bell and Laboudallon1986) then used simultaneous counts, also focused on the Vallée de Mai, to obtain a minimum population estimate of 58 individuals. Subsequent monitoring of C. (n.) barklyi coordinated by local ornithologist V. Laboudallon has been carried out in most years since 1985 via simultaneous counts from 24–50 vantage points. The results suggest that the population increased by c. 40% between 1985 and 1997, continued to grow until 1999 and then decreased until 2005 (Rocamora & Laboudallon, Reference Rocamora and Laboudallon2009). Rocamora & Skerrett (Reference Rocamora, Skerrett, Fishpool and Evans2001) estimated 200–300 individuals, and simultaneous counts in 2008 confirmed a minimum of 163 individuals, leading to a population estimate of 300–400 parrots (Rocamora & Laboudallon, Reference Rocamora and Laboudallon2009). In recent years simultaneous counts have not been as regular and the 2010 and 2011 counts were cancelled (V. Laboudallon, pers. comm.). Although simultaneous counts as an index of abundance are suitable for monitoring changes in population size over time and estimating the minimum population (Gregory et al., Reference Gregory, Gibbons, Donald and Sutherland2004), it is not known how this index relates to total population size. The most recent population estimate on Praslin, of 645 parrots (Walford, Reference Walford2008), used distance sampling at 39 random points but had several methodological and analytical constraints, which meant that assumptions of the distance sampling method were violated, resulting in a range that was considered too broad to serve as a basis for conservation planning.
Quantifying parrot abundance is not straightforward because they are often rare and patchily distributed, fly fast and for long distances, and inhabit dense forests with poor accessibility and visibility (Casagrande & Beissinger, Reference Casagrande and Beissinger1997). Common methods to estimate parrot populations are line transects, point counts and roost counts (Casagrande & Beissinger, Reference Casagrande and Beissinger1997). Mark–resighting methods are problematic because of the difficulties of capture. Territory mapping is only possible for territorial species. Simultaneous counts can be used to obtain minimum population estimates and generate an index of relative abundance but are not suitable for estimating absolute abundance (Gregory et al., Reference Gregory, Gibbons, Donald and Sutherland2004). For informed conservation decisions and assessment of conservation status, estimates of absolute population numbers are essential, particularly for a taxon with a restricted range.
A more accurate population estimate is a priority in the most recent Action Plan for the Seychelles black parrot (Rocamora & Laboudallon, Reference Rocamora and Laboudallon2009). In addition to facilitating conservation decisions, a reliable survey method needs to be established to ensure compatibility of future estimates and to maximize efficiency. The aims of this research are therefore to determine (1) the status of the Seychelles black parrot population across its range, with an updated and more precise population estimate, (2) whether there is a resident population of C. (n.) barklyi on Curieuse, (3) whether densities differ across habitat types, and (4) the most efficient count duration for future monitoring, and any other recommendations for conservation of the Seychelles black parrot.
Study area
The study was carried out on the islands of Praslin and Curieuse in the Seychelles archipelago in the Indian Ocean (Fig. 1). Praslin is the second largest of the granitic Seychelles islands, 44 km north of the largest granitic island, Mahé. The climate is tropical with little variation in monthly mean temperatures of 25–28 °C (Walsh, Reference Walsh and Stoddart1984). Humidity also varies little with season (monthly means of 75–80%) and annual rainfall is > 2,000 mm. Praslin usually experiences a dry season during May–October and a wet period during December–March (Walsh, Reference Walsh and Stoddart1984).
Praslin's c. 6,500 people live mainly near the coast. The coastal plain is relatively wide and heavily used for cultivation, residential areas, tourism and roads. The centre of the island is dominated by a ridge of hills rising to 367 m. Large areas of the island have been damaged by fire and are covered by secondary vegetation (Meuwly, Reference Meuwly2002). Few remnants of native palm forest remain in the uplands; the largest of these is protected in the Praslin National Park. Within the National Park lies the 19.5 ha Vallée de Mai, which has been managed and protected by the Seychelles Islands Foundation since 1989 and is the most popular visitor attraction in the Seychelles (Rist et al., Reference Rist, Kaiser-Bunbury, Fleischer-Dogley, Edwards, Bunbury and Ghazoul2010). The Vallée de Mai is the only site where all six palm species endemic to the Seychelles grow naturally, and it is dominated by the endemic coco de mer palm L. maldivica. L. maldivica is particularly important to C. (n.) barklyi as the majority of nests occur in dead trunks of this palm species and the Vallée de Mai is the parrot's main breeding site (Gaymer et al., Reference Gaymer, Blackman, Dawson, Penny and Penny1969; Evans, Reference Evans1979).
Curieuse (287 ha, maximum altitude 172 m), c. 1 km north of Praslin, is the only other island where L. maldivica occurs naturally. The vegetation is similar to that on Praslin's hilltops but Curieuse also supports patches of mixed forest and mangrove areas. Erosion and vegetation degradation have resulted from fire damage and the palm forest has been slow to recover. The L. maldivica dominated areas are relatively open, with low trees, unlike the closed canopy palm forest on Praslin.
Methods
Survey methods
Data were collected from December 2010 to March 2011. Point count distance sampling on Praslin and Curieuse was used to generate a population density estimate for C. (n.) barklyi. Based on Walford's Reference Walford2008 encounter rate (Walford, Reference Walford2008) it was estimated that a minimum of 242 point counts would provide a sufficient number of observations. To ensure sampling of all habitat types relative to their area we used a 500 × 300 m grid system spanning both islands (Fig. 1b), which resulted in 27 transects 500 m apart. Points were spaced every 300 m along transects, giving 268 survey points, 20 of which were located on Curieuse. Transects were surveyed in random order. The Curieuse points were all surveyed within 4 days (8–11 March 2011), with the random transect order maintained. Each point was visited once and surveys were only conducted in fair weather. Peaks in C. (n.) barklyi activity occur at 06.00–10.30 and 15.30–18.30 (Evans, Reference Evans1979, Seychelles Islands Foundation, unpubl. data) and all point counts were conducted between sunrise (06.00–06.20) and 10.30 h. We used an 8-minute point count period and started counts immediately on arrival, as recommended for canopy frugivores (Lee & Marsden, Reference Lee and Marsden2008). The data were recorded at 1-minute intervals to facilitate comparison of results for different count durations. The length of the sampling period was intended to minimize the risk of recording the same individual twice while maximizing encounter rates. Calls from a new location were only recorded as an additional observation if all previously heard parrots could still be heard in their initial locations (modified from Buckland et al., Reference Buckland, Marsden and Green2008). The timing of the sampling coincided with the breeding period. Breeding females remain relatively close to nest sites during this time and are highly vocal, which is advantageous for distance sampling as detection probability is maximized. To account for incubating females within nest cavities not being detectable, the immediate vicinity of the observation point was searched for potential nesting cavities following the count.
A global positioning system and aerial photographs were used to navigate on foot to within 20 m of planned census locations. For four points it was not possible to approach closer than 50 m (construction site, quarry and two points on airstrip). Point counts were always carried out by the same observer (AR), rotating around the point. Encounters were recorded as clusters of individuals, noting group size if known. For each encounter the distance to the centre of the group was recorded (Marsden & Pilgrim, Reference Marsden and Pilgrim2003). No upper distance limit was used but data were truncated at an appropriate distance during analysis. For each observation the method (visual/aural) and time of detection (by minute of count) were recorded.
We ensured that the extent to which three critical assumptions of the method (Buckland et al., Reference Buckland, Anderson, Burnham and Laake1993) were violated was minimized. Meeting the first assumption (that 100% of the individuals in close proximity to the observer are detected) was improved by searching within a 30 m radius of the census point for 5 minutes after the census period (Marsden, Reference Marsden1999; Marsden & Pilgrim, Reference Marsden and Pilgrim2003). Violation of the second (that objects are detected at their initial location) was minimized by recording at their point of origin all birds that were flushed by the observer approaching the census point (in addition to including birds that were initially perched). As a canopy-dwelling species, however, C. (n.) barklyi is not easily disturbed by ground-based observers. Observations of birds in flight were noted but excluded from the analysis (Marsden, Reference Marsden1999; Marsden et al., Reference Marsden, Whiffin, Sadgrove and Guimarães2000; Cahill et al., Reference Cahill, Walker and Marsden2006). Violation of the third assumption (that distances are recorded accurately) was minimized by measuring distances with a tape measure (< 10 m) and laser rangefinder (> 10 m) during or after the census period. Recommendations by Buckland et al. (Reference Buckland, Marsden and Green2008) were applied to obtain more accurate distance measurements of aural detections, and training in distance estimation of aural detections was carried out before and regularly alongside the population survey. Estimated distances made up only 5–10% of encounters.
On Curieuse point surveys were supplemented with additional watches. Areas with previous parrot sightings (Rocamora & Laboudallon, Reference Rocamora and Laboudallon2009; Seychelles Islands Foundation, unpubl. data) were targeted in addition to good vantage points. Watches of 2–5 hours were carried out in the afternoons and evenings and areas of cultivated fruiting trees were surveyed for signs of feeding parrots.
Habitat variables
Data were collected, by the same observer, to classify habitat into five broad categories based on expected detectability and previous knowledge of C. (n.) barklyi ecology. Habitat was assessed in a circle of 30 m radius around each sample point. Canopy height was estimated to the nearest metre and canopy cover by eye to the nearest 10%. Palm percentage was defined as the percentage of individual trees of the six endemic palm species and was recorded in four categories (0%; low, 1–33%; medium, 34–66%; high, 67–100%). The extent of native vegetation was recorded in two categories (majority native or majority exotic, corroborated by recording the three most common tree species). The five habitat types were defined as: (1) palm forest: high palm percentage, average canopy height ⩾ 6 m, canopy cover ⩾ 30%; (2) mixed forest: zero–medium palm percentage, canopy height ⩾ 6 m, canopy cover ⩾ 30%; (3) native scrub: canopy cover < 30% and/or canopy height < 6 m, majority of vegetation native; (4) exotic scrub: canopy cover < 30% and/or canopy height < 6 m, majority of plants exotic; (5) developed/cultivated: included residential areas, farmland and other land uses.
Statistical analysis
Distance 6.0 (Thomas et al., Reference Thomas, Buckland, Rexstad, Laake, Strindberg and Hedley2010) was used to estimate the population size; other analysis was carried out with R v. 2.10.1 (R Development Core Team, 2009). We used different sizes of intervals and truncation widths to fit the best population estimate model. Different models were explored and visual examination of all detection functions, Akaike information criterion, goodness-of-fit test and coefficients of variation (CV) were used to assess the model fit (Buckland et al., Reference Buckland, Anderson, Burnham and Laake1993). For all models CVs were calculated using the P3 estimator. Habitat was explored both as stratum and as covariate. As no parrots were detected on Curieuse, stratification by island was unnecessary and data were analysed separately for Praslin. The best model fit was achieved by a hazard rate detection function with cosine adjustment and habitat as covariate. This model used four equal intervals, a truncation width of 200 m and truncation for the calculation of cluster size at 50 m. The size bias regression function of Distance was used to correct for higher detectability of large clusters. We used separate analyses to produce habitat-, area- and count duration-specific estimates. For habitat estimates independent detection functions were used for each type, with the exception of a common function for both scrub habitats as there were insufficient detections of exotic scrub for a reasonable model fit. All other models used a global detection function and cluster size estimate.
To account for potential dependence of point counts within transects we ran two models, based on pooled counts within a transect and counts as independent points. As the resulting estimates, model fits and CVs did not differ between these analyses, we ran all models with independent points.
The influence of habitat characteristics on the probability of detecting parrots in a point count was modelled using a generalized linear model (with logit link, assuming quasi-binomial distribution of parrot presence) including the four explanatory variables (canopy height, canopy cover, palm percentage and percentage of native vegetation). We tested the variables for potential collinearity (using Spearman's rank correlation) and ran the model with all variables and without each correlated variable. All pairwise comparisons of habitat variables were significantly correlated except palm percentage with canopy height (P = 0.87) and percentage of native vegetation with canopy cover (P = 0.85). Because there was a statistically better fit of the model with all variables we used this model in our analysis. We used backwards model selection by removing non-significant explanatory variables to obtain the minimum adequate model. Population densities were compared across the five habitat types, between palm percentage classes and between inside and outside the National Park, using z-tests (Buckland et al., Reference Buckland, Anderson, Burnham, Laake, Borchers and Thomas2001). The resulting P-values were adjusted using the Benjamini & Hochberg (Reference Benjamini and Hochberg1995) procedure to compensate for multiple tests.
Results
A total of 181 observations of C. (n.) barklyi clusters were recorded across the 268 survey points, at distances of up to 400 m. No parrots were detected on Curieuse, even in surveys outside the point counts. After excluding the 23 (12.7%) detections of aerial birds and truncation at 200 m (which excluded 30 observations), 128 observations remained for analysis, 114 (89.1%) of which were aural, 13 (10.2%) were aural/visual and one (0.8%) was only visual. The encounter rate was 0.52 detections per point count on Praslin.
The estimated density of C. (n.) barklyi for Praslin is 0.14–0.24 individuals per ha, which gives a population estimate of 520–900 individuals (95% confidence interval). Most parrots were detected quickly, causing the number of encounters to increase steeply in the first 3–4 minutes of the count and then flatten off. Similarly, the density estimate and its CV approached an asymptote between 3 and 4 minutes, suggesting that a 4-minute count is sufficient to reach the level of accuracy achieved with the 8-minute count used.
Average parrot density in the National Park (0.44 ± SE 0.093 individuals per ha, n = 26) was higher than on the rest of the island (0.18 ± SE 0.090, n = 102, z = 2.99, P = 0.0014). Parrot detections on the coast were uncommon (0.28 ± SE 0.074 at points located < 300 m from the coast), the exception being the eastern part of the south coast where the palm forest almost reaches the shore (at least one parrot detection at all nine coastal points; Fig. 1b).
Almost half (44%) of Praslin was categorized as forest, with only a fifth of that being palm forest, the least common habitat type (Table 1; Fig. 1b). Parrot densities in forest habitats were generally higher than those in scrub habitats (Tables 1 & 2). They were highest in palm forest, followed by those in mixed forest (Table 1), although not all differences were significant (Table 2).
Table 1 Coverage of habitat types (in 30 m radius circles) on Praslin and Curieuse (Fig. 1), encounter rates of the Seychelles black parrot Coracopsis (nigra) barklyi, and density estimates in five different habitat types on Praslin.
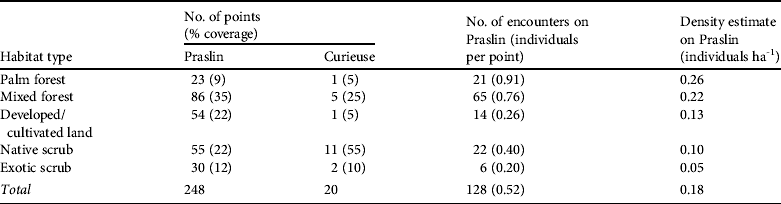
Table 2 Pairwise comparison (two-tailed z-tests, using Benjamini–Hochberg correction to adjust P-values for multiple testing) of estimated densities of the Seychelles black parrot in the five habitat types (in order of descending parrot densities).
*Adjusted P < 0.05; **Adjusted P < 0.001
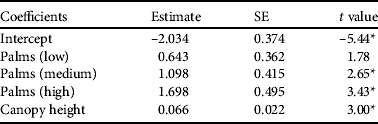
Parrots were more likely to be present in areas of high palm percentage and canopy height (P < 0.001; Table 3). Other habitat variables did not explain additional variation in parrot presence. Comparing parrot densities from different palm percentage classes confirmed that areas of high and medium palm occurrence had higher parrot densities than vegetation without palms (high–zero: z = 2.93, Padj = 0.01; medium–zero: z = 2.28, Padj = 0.034). The pairwise comparisons of other palm percentage classes did not differ (Padj > 0.05).
Table 3 Generalized linear model describing the influence of habitat variables on probability of detecting Seychelles black parrots during a point count. Palm category (low, middle, high) indicates the percentage of endemic palms in the vegetation. Non-significant variables excluded from the minimum adequate model were canopy cover and percentage of native vegetation.
* P < 0.01
Discussion
Status and range of the Seychelles black parrot population
We estimate the total population of Seychelles black parrots to be 520–900 in the wild. There is no evidence for a resident black parrot subpopulation on Curieuse and therefore the estimate for Praslin is the global total. Our estimate of population size is higher than that of most previous surveys but falls within the range of the most recent previous survey (Walford, Reference Walford2008). The search effort in obtaining the estimate presented here was higher than that of both the simultaneous counts (Rocamora & Skerrett, Reference Rocamora, Skerrett, Fishpool and Evans2001; Rocamora & Laboudallon, Reference Rocamora and Laboudallon2009) and previous surveys in the Vallée de Mai (Gaymer et al., Reference Gaymer, Blackman, Dawson, Penny and Penny1969; Evans, Reference Evans1979). The methods used in these surveys were also fundamentally different, and thus our higher population estimate is not directly comparable to the earlier results. Comparing our results to those from simultaneous counts conducted during the same period as our study would facilitate retrospective estimates of total population size from previous counts. An increase in population size over the past 40 years seems likely (Rocamora & Laboudallon, Reference Rocamora and Laboudallon2009) given the parrot's increased range on Praslin. Previous authors reported the majority of the population living in the Vallée de Mai (Gaymer et al., Reference Gaymer, Blackman, Dawson, Penny and Penny1969; Evans, Reference Evans1979) whereas we regularly observed parrots (and breeding attempts) outside the Vallée de Mai, and estimated population densities were locally almost as high beyond as within the valley. Legal protection (granted to the parrot in 1966 and to all endemic palms in 1991) and expansion of fruit tree plantations on Praslin may have contributed to this increase in the parrot population.
If confirmed as a distinct species endemic to the Seychelles, C. (n.) barklyi would require IUCN Red List inclusion separate to the C. nigra sub-species of Madagascar and the Comores. With no evidence of a recent decline only criterion D of the IUCN Red List criteria (IUCN, 2001), for small population sizes and areas of occupancy, applies. Criteria D1 (‘population size estimated to number fewer than 1,000 mature individuals’) and D2 (‘very restricted … number of locations (typically five or fewer)’) for categorization as Vulnerable are fulfilled.
Curieuse has only ever been considered to harbour a small sub-population of parrots (Rocamora & Laboudallon, Reference Rocamora and Laboudallon2009) but the confirmation that Curieuse does not and probably cannot support a resident population highlights the vulnerability of the parrots on Praslin to potential catastrophes such as disease (e.g. the only Psittacula echo population in Mauritius was threatened by a sudden outbreak of Psittacine Beak and Feather Disease; Malham et al., Reference Malham, Kovac, Reuleaux, Freeman, Linnebjerg and Kennerly2007) or extensive forest fire. The parrot's range, however, now encompasses most of Praslin, which will help to buffer the population against stochastic events.
Parrot densities across habitat types
Both the habitat type comparison and the analysis of the factors that influence parrot presence showed that forest habitats were used more frequently than scrub and that the presence of palms enhances habitat suitability. Palm forest supported the highest densities of parrots and is known to be important feeding habitat (Rocamora & Laboudallon, Reference Rocamora and Laboudallon2009; Reuleaux, Reference Reuleaux2011). Our results indicate that the National Park, particularly the Vallée de Mai, is the core area for the subspecies. The pattern of significant differences in parrot densities only between pairs of habitats with large sample sizes, suggests that low sample size may explain the lack of differences between some of the other pairs: e.g. the scarcer palm forest had few point counts thus, despite hosting the highest parrot encounter rate and density, differences in density compared to habitat types with intermediate encounter rates were not significant. Although our results show the importance of native palms for the parrot, we did not find an influence of native vegetation per se on parrot presence, which might represent a real lack of influence but is probably because of our simplified evaluation of native vegetation in only two categories.
Recommendations for survey methodology
There has been little research on the effect of the duration of the count on density estimates derived with Distance. Our results indicated that approximately the same density estimate was achieved with a 4-minute as with an 8-minute interval. A count duration of 5 minutes would therefore be sufficient to detect a decrease in new encounters in the last minute, confirming that few detections will be missed by reducing the count duration. A similar pattern was observed by Lee & Marsden (Reference Lee and Marsden2008). Cimprich (Reference Cimprich2009) found that shorter count periods (using only aural detections) produced lower, more accurate estimates, as verified by territory mapping, in a comparison of 3-, 5- and 6-minute counts of black-capped vireos Vireo atricapilla. This species is very active and vocal and tends to move between songs, which increases the risk of double counting. Although our study also largely relied on aural cues, C. (n.) barklyi does not move as frequently as a passerine and often calls in flight (Gaymer et al., Reference Gaymer, Blackman, Dawson, Penny and Penny1969; Reuleaux, Reference Reuleaux2011), which alerts the observer to the change of position.
For future monitoring of C. (n.) barklyi we recommend (1) using a 5-minute interval to obtain a similar level of accuracy to the estimate presented here, (2) repeating the survey every 5–10 years, or following signs of population decline, to monitor population status and trends, (3) excluding the 30 points on Curieuse and the 20 points in exotic scrub, if there are time constraints, unless birds are discovered breeding on Curieuse, and (4) applying the methods described here to ensure comparability of estimates. Additionally, the existing ringing programme should be extended to enable individual monitoring, survival analysis and verification of population estimates.
Conservation
To improve the assessment and mitigation of threats to parrots generally, Collar (Reference Collar2000) recommended further population surveys and ecological research. With a current minimum population of > 500 birds, we recommend further ecological research prior to implementing proposed intensive conservation measures (e.g. translocations to other islands, nest site manipulations, provision of artificial nest sites, as proposed in Rocamora & Laboudallon, Reference Rocamora and Laboudallon2009). Results from our research and from the Seychelles Islands Foundation monitoring programme suggest that such interventions will not be necessary in the short term. Rather, continued research into breeding ecology and expansion and improvement of the parrot's endemic palm forest habitat on Praslin would be the most effective course of action for its conservation. This could be expanded to include habitat restoration on other islands (e.g. Silhouette Island), where the parrot has not been documented to occur historically but which would be well suited to support a viable backup population to buffer the risk of extinction. Categorizing the Seychelles black parrot as Vulnerable on the IUCN Red List would highlight the fragility of this island endemic and promote future research on and conservation management for this flagship species and its unique palm forest habitat.
Genetic and morphological research on the taxonomic status of the C. nigra subspecies is currently underway. Pending these results our recommendations will be passed to IUCN. A ringing scheme is ongoing, with 112 individuals ringed, but the majority of incidental sightings are still of unmarked parrots. Depending on progress with ringing more precise population surveys may be possible, but failing this the census will be repeated by the Seychelles Islands Foundation, using the methods recommended here, in 2015. In the meantime the simultaneous counts have been re-initiated by the Environment Department, which will provide comparative data on the localized population trends of this island parrot.
Acknowledgements
We thank the Seychelles Islands Foundation and Vallée de Mai staff, particularly Frauke Fleischer-Dogley, Marc Jean-Baptiste, Marcus Pierre, Wilna Accouche, Nathachia Pierre and Uzice Samedi, the many Praslinois who permitted access to land, including the Seychelles National Parks Authority, Praslin Development Fund, Coco de Mer Hotel, Château de Feuilles, Emerald Cove, Lemuria Hotel and Iles des Palmes, the Global Vision International team for their support on Curieuse, the Seychelles Bureau of Standards and Seychelles Department of Environment for approving and supporting the research, and Christopher Kaiser-Bunbury and Sacha Viquerat for statistical advice. We are grateful to Stuart Marsden and an anonymous referee for reviewing and improving this article. The study was funded by the German Academic Exchange Service (DAAD) and the Gesellschaft für Tropenornithologie (GTO). MW is currently supported by a grant from Volkswagen Foundation, Germany.
Biographical sketches
Anna Reuleaux researches the ecology of the Seychelles black parrot on Praslin. She previously worked on conservation management in Mauritius. Nancy Bunbury coordinates research and conservation projects for the Seychelles Islands Foundation at the two UNESCO World Heritage Sites in the Seychelles: the Vallée de Mai and Aldabra Atoll. Pascal Villard has been studying the ecology of island bird species since 1993 in the Caribbean, Pacific and Indian Ocean. Matthias Waltert studies the effects of forest use on tropical biodiversity and teaches wildlife population and biodiversity assessment on the integrated bi-national MSc on International Nature Conservation at Georg-August-University Göttingen, Germany, and Lincoln University, New Zealand.






