Introduction
The decline of populations of scavenging raptors, notably vultures and kites, is emerging as a serious phenomenon. Most dramatic has been the loss of > 90% of Gyps vultures in the Indian subcontinent as a result of accidental diclofenac poisoning (Pain et al., Reference Pain, Bowden, Cunningham, Cuthbert, Das and Gilbert2008). However, this extraordinary circumstance has overshadowed events elsewhere, although in some respects these are more worrying because they are less obviously remediable. There have been steep declines in South-East Asia’s vulture populations (Pain et al., Reference Pain, Bowden, Cunningham, Cuthbert, Das and Gilbert2008; J. W. Duckworth et al., unpubl. data). West Africa has lost 98% of its large vultures outside protected areas within 30 years (Thiollay, Reference Thiollay2006). Greece lost 84% of its bearded vultures Gypaetus barbatus within 10 years (Xirouchakis & Tsiakiris, Reference Xirouchakis and Tsiakiris2009). The Egyptian Neophron percnopterus and red-headed vulture Sarcogyps calvus were categorized as Endangered and Critically Endangered, respectively, on the IUCN Red List in 2007, following assembly of the evidence (Cuthbert et al., Reference Cuthbert, Green, Ranade, Saravanan, Pain, Prakash and Cunningham2006). Across southern Europe the red kite Milvus milvus is declining in all countries where it occurs (BirdLife International, 2009). The black kite Milvus migrans, although generally widespread and common, is also showing signs of decline (Sergio et al., Reference Sergio, Pedrini and Marchesi2003). The deteriorating status of these species seems primarily to be linked to incidental mortality from feeding on carcasses deliberately poisoned to control mammals regarded as pests, although direct persecution, disturbance to breeding birds and diminution of food supplies are also believed to play a part (see, for example, accounts of scavengers in Tucker & Heath, Reference Tucker and Heath1994).
Raptors in Cape Verde comprise seven species: black kite, red kite, Egyptian vulture and common buzzard Buteo buteo (Accipitridae), osprey Pandion haliaetus (Pandionidae), and common kestrel Falco tinnunculus and peregrine Falco peregrinus (Falconidae). Four of these species occur as five endemic subspecies, fasciicauda (red kite), bannermani (common buzzard), neglectus and alexandri (common kestrel) and madens (peregrine), all of which Hazevoet (Reference Hazevoet1995) elevated to species under the phylogenetic species concept but which we here retain as subspecies. Three of these species, the two kites and vulture, may be typified as scavengers, as this is their primary mode of foraging (all take live prey opportunistically), although the buzzard often also scavenges; the others exclusively take live prey. All seven are represented strongly in Europe, and the Cape Verde populations of osprey, red kite, common buzzard and common kestrel are the south-westernmost outliers of their Palaearctic ranges, and there are Afrotropical breeding populations of black kite, Egyptian vulture and peregrine.
The vulnerability of scavengers has been detected in a general analysis of avian susceptibility to environmental change, with a prediction of relatively high losses of members of this guild in the 21st century (Sekercioglu et al., Reference Sekercioglu, Daily and Ehrlich2004). Using the Cape Verde raptor assemblage as a case study we examine whether the patterns of population change in the archipelago’s seven species confirm other evidence that scavengers are faring worse than birds that catch live prey.
Methods
For each species we synthesized relevant information (status assessments and individual records) from all available literature, published and unpublished, and arranged it by species and island (13 in the Cape Verde archipelago, with Ilhéus do Rombo treated as one island: Fig. 1). We added unpublished information from fieldwork by SMH in 1996–2003 involving (1) a 1997 census of osprey and Egyptian vulture on Santo Antão, São Vicente, Boavista, Sal, Brava, Fogo and Maio; (2) a 2001 census and territory mapping of all raptors across Santo Antão in which carrion feeders were lured with goat carcasses; (3) a 2001 census of all raptors on Santa Luzia, Raso and Branco (with Raso censused again in 2003); (4) a 2002 month-long survey of kites on Maio and Boavista; (5) an intensive search for all nest sites of common kestrels in 1996–2001 in which 30% of every island’s surface, representing the main environmental units (desert, agricultural field and settlements), were covered (only breeding pairs counted), with extrapolation of total nests based on proportions of these units per island. Because habitat quality varies strongly both between and within islands we did not calculate population densities for each species per island.
Fig. 1 The Cape Verde archipelago. The inset shows the location of the archipelago off the west coast of Africa.
We then filtered this material for highest abundance (often qualitative, especially in accounts by earlier visitors) and most recent abundance. We used these two values to tabulate change over time and classified populations crudely into three status conditions: common (taxa that, according to statements in the literature or to observations by SMH, can or could regularly be seen in most parts of a given island), rare (taxa not numerous and restricted to certain areas of an island), and extinct, which includes vagrants (the legend to Table 1 provides further definitions of these categories). We then assessed status trends by depicting changes between these three status conditions by island and species: no change was classed as such, a one-category change (e.g. common to rare) was classed as a decline, and a two-category change (e.g. common to extinct) was classed as severe decline. Each island was assigned one of these trend categories to show the proportion of trend categories per species.
Table 1 Distribution and population trends of Cape Verde raptors by island, setting highest reported abundance against most recent evidence. Each species has an upper (highest numerical value) and lower (latest numerical value) row. Under each island a number or code (abd, abundant; br, breeding; com, common; n/r, no record; num, numerous; p, pairs; pres, present; unk, unknown; wds, widespread; ?, situation uncertain), with superscript reference or comment, is followed by a status letter (C, common: generally a number of descriptions suggesting island well stocked, interpreted as easy to find; R, rare: generally < 5 individuals, interpreted as hard to find; X, extinct; X?, situation uncertain: rarely present, vagrant, not proven to breed, likely to be inviable or probably extinct). For common kestrel: n, subspecies neglectus; a, subspecies alexandri.
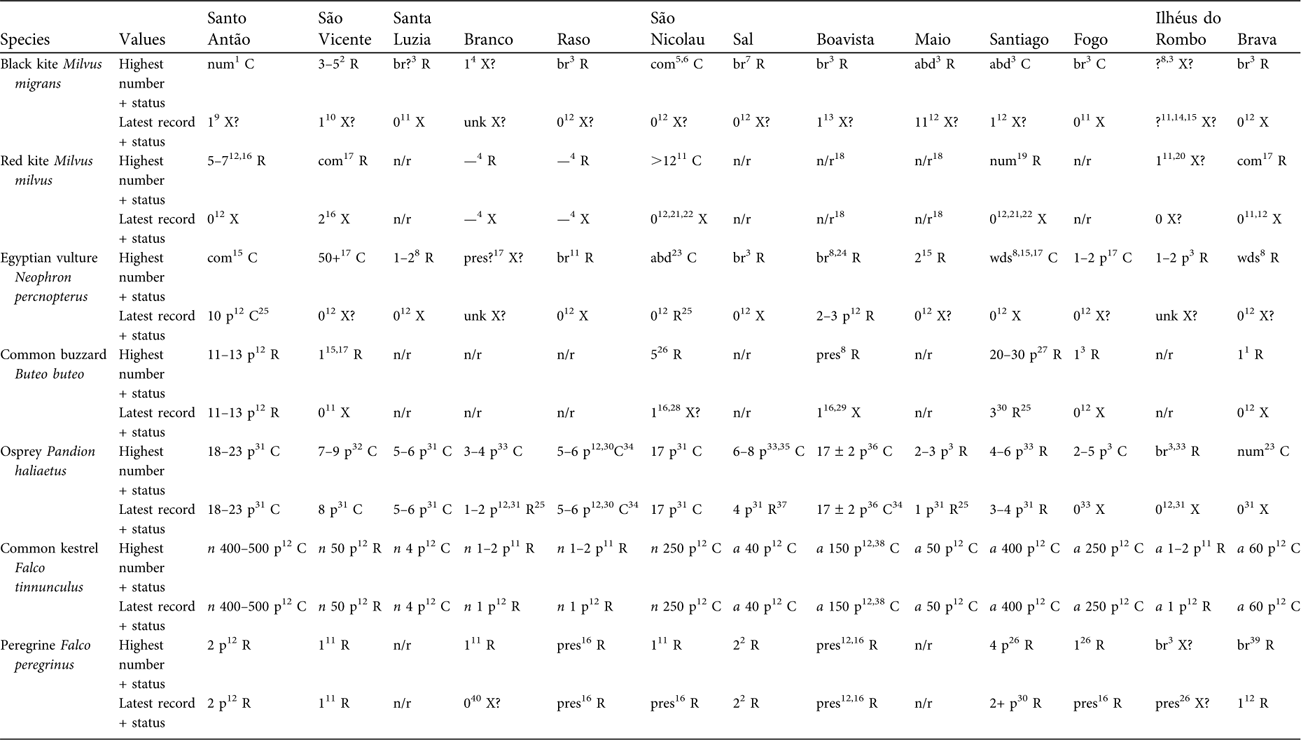
1Nørrevang & den Hartog (Reference Nørrevang, Hartog and Lobin1984). 2Hazevoet (Reference Hazevoet1998). 3de Naurois (Reference Naurois1969a). 4Supposedly reported by early authors fide Bourne (Reference Bourne1955) but documentation unclear. 5Interpreted here as this species, because reported to take poultry and catch fish at the coast (Keulemans, Reference Keulemans1866), although interpreted by Hazevoet (Reference Hazevoet1995) as red kite. 6Keulemans (Reference Keulemans1866). 7de Naurois (Reference Naurois1987a). 8Alexander (Reference Alexander1898b). 9Carter (Reference Carter2001). 10van der Molen (Reference Molen2008). 11Hazevoet (Reference Hazevoet1995). 12SMH personal records. 13Breider et al. (Reference Breider, Forsberg, Svensson, Ullman and Wigermo2004). 14No indication of numbers but all assumed to be visitors only. 15Bannerman (Reference Bannerman, Bannerman and Bannerman1968). 16Hazevoet (Reference Hazevoet2003). 17Bourne (Reference Bourne1955). 18Hille & Collar (Reference Hille and Collar2009). 19Summers-Smith (Reference Summers-Smith1984). 20Presumed vagrant, 1897 (Hazevoet, Reference Hazevoet1995). 21Hille (Reference Hille1998). 22Hille & Thiollay (Reference Hille and Thiollay2000). 23Bolle (Reference Bolle1856). 24Salvadori (Reference Salvadori1899). 25Apparently with a 50% decline. 26Anderson & White (Reference Anderson, White, Chancellor and Meyburg2000). 27de Naurois (Reference Naurois1973). 28Hazevoet (Reference Hazevoet1997). 29Attribution of genetic material to bird from Boavista (Kruckenhauser et al., Reference Kruckenhauser, Haring, Pinsker, Riesing, Winkler, Wink and Gamauf2004) is a mistake for Santo Antão (SMH). 30Krabbe et al. (Reference Krabbe, Elias and Riley2003). 31Palma et al. (Reference Palma, Ferreira, Cangarato and Vaz Pinto2004). 32Ferreira & Palma (Reference Ferreira, Palma, Chancellor and Meyburg2000). 33de Naurois (Reference Naurois1987b). 34Apparently 50% increase. 35de Naurois & Bonnaffoux (Reference Naurois and Bonnaffoux1969). 36Lopes Suarez & Varo Cruz (Reference Lopes Suarez and Varo Cruz2007). 37Apparently 40% decline. 38Estimate of 77 (Ontiveros, Reference Ontiveros2005) was derived from inappropriate methodology and analysis. 39de Naurois (Reference Naurois1969b). 40den Hartog (Reference Hartog and Lobin1990).
Results
Scavenging raptors in Cape Verde have declined more steeply and across more islands than predatory raptors, with the partial scavenger, common buzzard, showing an intermediate decline between the mainly scavenging and the purely predatory species (Fig. 2; Table 1). Degree of change in status varies between islands, with Santo Antão offering the best prospects for conservation (Fig. 3). The literature and our data provide further more generalized evidence on the status of individual species.
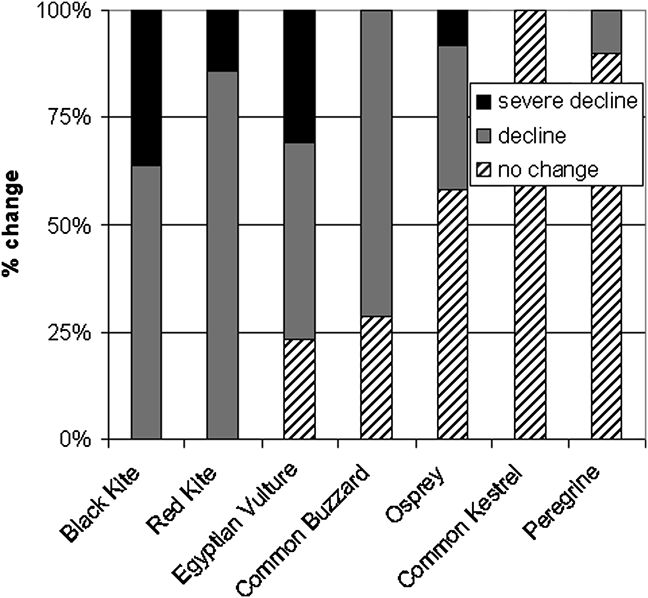
Fig. 2 Trends in populations of the seven raptor species across 13 Cape Verde islands (Fig. 1). Each island is assigned a trend category (severe decline, decline, or no change) for each species, and columns show the proportion of each trend category per species. No change indicates populations with R–R and C–C but not X?–X? (abbreviations defined in Table 1) because no viable population was recorded. C–R, R–X and R–X? (one-category changes) indicate decline, and C–X and C–X? (two-category changes) indicate severe decline.
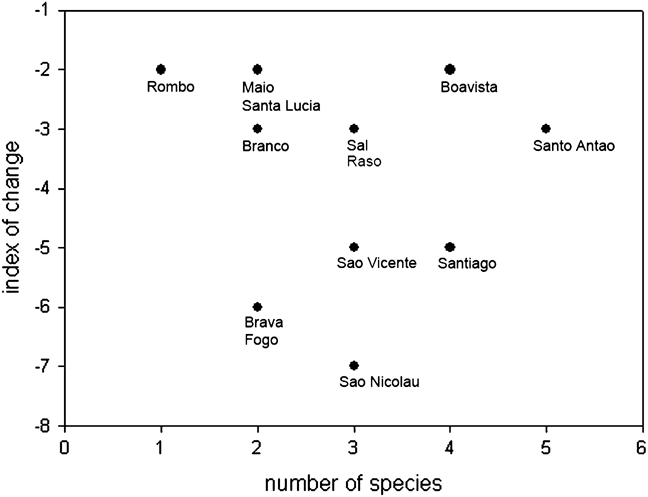
Fig. 3 Population trend and number of raptor species by island in Cape Verde. The index of change sums all changes for all species per island, obtained by subtracting 1 for one step down and 2 for two steps down between the categories severe decline, decline and no change (explained in Fig. 2). Santo Antão has the most species but shows the second slowest rate of deterioration, and is thus a conservation priority.
Black and red kites Early visitors did not realize that both species, which are very similar, were present, so the record up to the 1950s is confused, with black kite being particularly poorly documented (Hille & Collar, Reference Hille and Collar2009). Despite ‘apparently identical ecologies’ (Naurois, Reference Naurois1969a), the two may have diverged by habitat, with red kite more in mountains and black kite more on coasts (Hartog, Reference Hartog and Lobin1990), which might explain why fasciicauda perhaps never occurred on low-lying Sal, Boavista and Maio (Hazevoet, Reference Hazevoet1995). However, the future of both species in Cape Verde is in doubt, with the black kite suffering a greater spread of severe declines than any other raptor (Fig. 2) and the red kite extinct or near-extinct (Table 1; Hille & Collar, Reference Hille and Collar2009). The last red kites seen on Santo Antão, in the 1990s, were chasing young chickens in the mountains, hunting big grasshoppers and patrolling harbours, seeking food even near houses but always at a distance from people (SMH, pers. obs.). The population of the red kite may never have numbered > 100. In the late 1980s it was guessed to be 50–100 birds (including hybrids) and in the mid-1990s ‘some tens of pairs’ (Ferguson-Lees & Christie, Reference Ferguson-Lees and Christie2001) but it is no longer certain that any survive (Hille & Collar, Reference Hille and Collar2009).
Egyptian vulture This species ranges in dry open country from shoreline to high mountains (Hazevoet, Reference Hazevoet1995; SMH, pers. obs.). Cape Verde was not colonized by humans and their livestock until 1460 and never had an indigenous non-volant mammal, so any pre-settlement vulture must have subsisted on seabird and turtle colonies (Hartog, Reference Hartog and Lobin1990); Naurois (Reference Naurois1969a) noted that the species could catch grounded petrels near their burrows. Its scavenging habits earned it both legal protection (Bolle, Reference Bolle1856) and popular support (Bannerman, Reference Bannerman, Bannerman and Bannerman1968) and in the early 1950s it was ‘abundant around the large towns’ except on Fogo and Brava, with ‘one or two pairs around most villages’ (Bourne, Reference Bourne1955). In the 1960s Naurois (Reference Naurois1969a) reported that it ‘swarms’ (pullule) in the archipelago. However, by 1986–1993, never more than five were seen together anywhere except on Santo Antão (Hazevoet, Reference Hazevoet1995) and the species was ‘declining at an alarming rate’ (Hazevoet et al., Reference Hazevoet, Fischer and Deloison1996).
Common buzzard Largely found in remote montane areas, this typically soaring raptor, which takes both live and dead prey, went unrecorded by most pre-1950 explorers, only one of whom referred to it as ‘not uncommon’ (Bannerman, Reference Bannerman, Bannerman and Bannerman1968). Hazevoet (Reference Hazevoet1995, Reference Hazevoet1998, Reference Hazevoet2003) described it as ‘exceedingly rare in last few decades’, with a total population of ‘some tens of pairs’, and our analysis suggests declines on over half the islands. Records from Boavista comprise two specimens and one recent sighting: James (Reference James1984) attributed the first specimen to long-legged buzzard B. rufinus cirtensis, and Hazevoet (Reference Hazevoet1995, Reference Hazevoet2003) considered the second very similar to the first, so it is conceivable that the tiny population on this easternmost island actually involves a different species.
Osprey This all-year resident feeds exclusively on marine fish and nests from beaches to high mountains (SMH, pers. obs.). Bolle (Reference Bolle1856) recorded it on ‘all 10 islands’ and as ‘especially numerous on the eastern flatter sides where the sea is richest in fish’ (our translation). Keulemans (Reference Keulemans1866) also described it as numerous. However, Alexander (Reference Alexander1898b: 100) and the collectors on the Blossom (early 1920s) found it absent or very rare in the southern islands (Bourne, Reference Bourne1955); a circumstance confirmed by SMH and Palma et al. (Reference Palma, Ferreira, Cangarato and Vaz Pinto2004). Naurois (Reference Naurois1969a) estimated c. 50 pairs in the 1960s, which he considered reflected a decline attributable to human nest despoliation. Hazevoet (Reference Hazevoet1995) also estimated c. 50 pairs in 1988–1993, with 3–8 pairs on each island, 1–2 pairs on each islet and largest numbers on Santo Antão and the three eastern islands. Surveys in 1996–1997 (SMH, pers. obs.) and 1998–2001 (Ferreira & Palma, Reference Ferreira, Palma, Chancellor and Meyburg2000; Palma et al., Reference Palma, Ferreira, Cangarato and Vaz Pinto2004) resulted in estimates of pairs of 55–65 (SMH, pers. obs.), 44 or perhaps 60 (Ferreira & Palma, Reference Ferreira, Palma, Chancellor and Meyburg2000), 76–86 (Barone et al., Reference Barone, Delgado and Fernández del Castillo2000) and 72–81 (Palma et al., Reference Palma, Ferreira, Cangarato and Vaz Pinto2004). Accessible sites in the 1960s and earlier have been abandoned and the species only survives in remoter areas (SMH, pers. obs.; Ferreira & Palma, Reference Ferreira, Palma, Chancellor and Meyburg2000).
Common kestrel Two endemic subspecies inhabit rocky areas with slopes and updraughts. On the southern islands in 1897 ‘numerous pairs [could] frequently be found in one [palm] grove’ (Alexander, Reference Alexander1898a), something not observed today (SMH, pers. obs.). Bannerman (Reference Bannerman, Bannerman and Bannerman1968) believed numbers were ‘declining at a rapid rate’ in the 1960s but intensive surveys on all islands in 1996–2001 (Hille et al., Reference Hille, Nesje and Segelbacher2003) suggested no significant status change, yielding totals of 706 pairs of neglectus and 951 pairs of alexandri (SMH, pers. obs.; Table 1).
Peregrine Breeding was only proved in 1963 for this inhabitant of high cliffs and mountains on coasts and in island interiors (Bannerman, Reference Bannerman, Bannerman and Bannerman1968; Naurois, Reference Naurois1969b). Naurois (Reference Naurois1969a) thought it might be almost extinct and later argued, dubiously, that numbers must have been higher 60 years earlier, given the ease with which the Blossom expedition obtained specimens (Naurois, Reference Naurois1984). Hazevoet (Reference Hazevoet1995) thought it probably occurred ‘in very small numbers on all islands and islets’, perhaps < 20 birds, later suggesting ‘a few tens of pairs’ (Hazevoet Reference Hazevoet1997, Reference Hazevoet2003). There has been virtually no status change.
Discussion
The evidence from Cape Verde indicates that scavenging is a strategy in which the balance of risk and advantage is rapidly lost under the influence of modernized societies (Fig. 2). Black and red kite populations have declined on all islands to the point of near-extinction. The Egyptian vulture has also suffered widespread declines, with only two populations, on Boavista and Santo Antão, categorized as no change (i.e. remaining in the category common), although the former is tiny and the latter has declined by 50%. The part-scavenging common buzzard showed a decrease on > 70% of islands, and only Santo Antão and Santiago harbour small but stable (no change) populations, although again the latter declined by > 50%. Ospreys also exhibited declines but continue to flourish in the north-west. Only common kestrel (both subspecies, all populations) has not changed in status, and nearly all peregrine populations remained stable.
Raptor extinctions on Mediterranean and Macaronesian islands have been shown to be more likely where human populations are denser, probably because of persecution, and species dependent on human activities are also more liable to extinction (Donázar et al., Reference Donázar, Gangoso, Forero and Juste2005). In Cape Verde the human population density increased from 36 km-2 in 1950 to 109 km-2 in 2000 (UN Department of Economic and Social Affairs, 2008). We outline the three major threats to raptors on the islands, in their likely order of impact.
Pesticides and poisons Hazevoet et al. (Reference Hazevoet, Fischer and Deloison1996) and Hazevoet (Reference Hazevoet1998, Reference Hazevoet1999) believed the decline of the red kite and common buzzard to be a side effect of the relatively modern local practice of poisoning feral dogs with laced meat, the birds eating either the bait or the corpses and, if not succumbing directly, then probably suffering decreased fertility. This problem also afflicts the Egyptian vulture, two bodies of which were found beside a dead cat at a Santo Antão dump in 2000 (SMH, pers. obs.). Rodenticides and insecticides may, however, also have significant effects. Pesticide use on the islands is common and includes organophosphorous substances. Farmers use carbamates as insecticides, nematocides, DDT, strychnine and rodenticides (e.g. Raticide, Racumin and Fostrien; SMH, pers. obs.). Fostrien was widely used on Santiago at least until the 1990s, and methyl parathion (E 601), which poses a higher risk to birds than to other vertebrates (Grue et al., Reference Grue, Fleming, Busby and Hill1983; Mañosa et al., Reference Mañosa, Mateo, Freixa and Guitart2003), has been used against grasshoppers in crop fields on some islands, including Boavista (SMH, pers. obs.).
Persecution Although Cape Verde children tend to persecute all raptors (SMH, pers. obs.), the two falcons and vulture generally escape human interference: the kestrel is harmless, the peregrine reclusive and the vulture useful (which perhaps helps explain why the vulture has fared slightly better than the other scavengers; Fig. 2). However, the two kites and buzzard have always attracted hostility as chicken thieves (Alexander, Reference Alexander1898b; Bannerman, Reference Bannerman, Bannerman and Bannerman1968); even in the 1990s black kites would be pursued with stones (SMH, pers. obs.). Kite nests were plundered, explaining the black kite’s near-disappearance from São Vicente (Bannerman, Reference Bannerman, Bannerman and Bannerman1968). Keulemans (Reference Keulemans1866) reported that local people regarded kite meat (especially nestlings) as a delicacy. The tradition has persisted, although interviewees today report disliking the flavour (Hille, Reference Hille1998; SMH, pers. obs.). Eggs and young of ospreys were also considered delicacies: Naurois (Reference Naurois1969a, Reference Naurois1984) blamed the species’ decline on this, as did Ferreira & Palma (Reference Ferreira, Palma, Chancellor and Meyburg2000) for the decline on Sal, and thought the birds only escaped by nesting on inaccessible cliffs.
Food supply decrease Natural fluctuations in food supplies, caused by periodic drought, are common in Cape Verde, and predator populations fluctuate in response. Darwin’s failure to record a single Egyptian vulture while prospecting Santiago in 1832, and breeding inactivity in common buzzards on Santiago in 1969, were attributed to drought-induced loss of, respectively, livestock (Dohrn, Reference Dohrn1871; Bannerman, Reference Bannerman, Bannerman and Bannerman1968) and rodents (Naurois, Reference Naurois1969a). However, in the past 150 years overall food availability has probably declined. Lower rainfall caused by Saharan expansion (Rosenfeld et al., Reference Rosenfeld, Rudich and Lahav2001) and overgrazing by, and harvesting wild plants as fodder for, goats, which increased in numbers from 40,000 in 1961 to 148,000 in 2004 (República de Cabo Verde, 2004), must have produced much sparser, less productive vegetation. Invertebrate density has been thinned in farmed areas. Changes in livestock management (fewer available corpses and afterbirths), food resourcing (beef imported rather than reared locally), slaughterhouse practices (less waste), refuse disposal patterns (use of burning) and sanitation regimes (cleaner streets and flush toilets) have resulted in fewer carcasses, rodents and faeces (Bannerman, Reference Bannerman, Bannerman and Bannerman1968; Hazevoet, Reference Hazevoet1995). There is no direct evidence of the impact of these various constraints but all five black kites caught on Maio in 2002 (Hille & Collar, Reference Hille and Collar2009) showed stress bars in tail and wing feathers (SMH, pers. obs.).
Santo Antão, with its high agricultural output, permanent streams and comparatively low human density, is the stronghold of all raptor taxa in Cape Verde except the kites (Fig. 3). Its northern valleys, notably Ribeira da Torre, Ribeira Grande and Ribeira do Paul, harbour the last significant buzzard and vulture populations, and the highest densities of kestrels, in Cape Verde, and ospreys nest there close to the sea. Cape Verde is an Endemic Bird Area (Stattersfield et al., Reference Stattersfield, Crosby, Long and Wege1998) but although 12 Important Bird Areas have been identified there (Hazevoet, Reference Hazevoet, Fishpool and Evans2001), none is on Santo Antão. A protected area, with so-called restaurants to provide safe food for scavengers, as advocated in Spain by Hernández & Margalida (Reference Hernández and Margalida2009), coupled with a public awareness campaign, would be a valuable conservation measure in the island’s northern valleys, especially in light of the vulture and buzzard declines on the island, which indicate that land-use change and agricultural and other human practices continue to have a negative impact.
The plight of scavenging raptors in Cape Verde shows that this guild is significantly disadvantaged by human behaviour and economic development. It also points to the general conservation problem of scavengers on islands elsewhere in Macaronesia and the Mediterranean as a result of their naturally small populations and low rates of dispersal (Eurasian griffons, for example, have great difficulty crossing water; Bildstein et al., Reference Bildstein, Bechard, Farmer and Newcomb2009). There is, moreover, emerging evidence that these populations have been isolated long enough to have developed into distinguishable demes and taxonomic entities. The Egyptian vultures on the Canaries are morphologically, genetically and hence taxonomically distinct (Donázar et al., Reference Donázar, Negro, Palacios, Gangoso, Godoy and Ceballos2002; Kretzmann et al., Reference Kretzmann, Capote, Gautschi, Godoy, Donázar and Negro2003), and even the seemingly undifferentiated black kite of Cape Verde possesses certain minor but clear morphological characters (Hille & Collar, Reference Hille and Collar2009). We infer from these pieces of evidence that insular populations of scavenging raptors may represent locally adapted forms that cannot easily be replaced and therefore require targeted intervention to ensure their survival. Greater control of toxic materials, broad public awareness campaigns and the use of restaurants, which are also relevant to many continental populations, are crucial in this regard.
In October 2010 the European Union, after stalling for some years owing to public health considerations relating to TSE (transmissible spongiform encephalopathies), gave its approval to the use of feeding stations for scavenging raptors in all relevant range states (EC regulation 1069/2009). This welcome news unfortunately does not extend to Cape Verde, and the next step there will be to negotiate for a major landscape management initiative that will stabilize and improve the status of the country’s most vulnerable raptors.
Acknowledgements
SMH’s fieldwork was funded by the German Ornithological Society, European Commission (Marie Curie grant) and Peregrine Fund. We thank Roy Dennis, Simon Thomsett, Jim Wilmwarth, Sandra Krätschmer, Ute Nau, Armin Nemitz and Gernot Segelbacher for help in the field, Maria Tereza Vera Cruz and Hans-Uwe Heckel for logistic support, K.L. Bildstein for helpful information, and J.A. Donázar and V. Bretagnolle for valuable comments.
Biographical sketches
Sabine M. Hille studies the evolution and dynamics of small bird populations on islands and in changing environments. She has been doing research in Cape Verde for several years. Nigel J. Collar works on threatened bird species, running projects in Angola and Ethiopia.






