Introduction
Amazon River dolphins Inia geoffrensis are aquatic mammals occurring in the Amazon–Orinoco basin of Brazil, Colombia, Ecuador, Peru and Venezuela. They are distributed across all aquatic habitats, including backwaters, small lagoons, confluences and flooded forests. Their habitat use is influenced significantly by flood pulses, as the quantity and quality of available habitats change annually (McGuire & Winemiller, Reference McGuire and Winemiller1998; Martin & da Silva, Reference Martin and da Silva2004b; Gomez-Salazar et al., Reference Gomez-Salazar, Trujillo, Portocarrero-Aya and Whitehead2012). In a regional study, lakes, confluences and areas within 200 m of riverbanks were identified as critical habitat hotspots with the highest dolphin densities (Martin & da Silva, Reference Martin and da Silva2004b; Gomez-Salazar et al., Reference Gomez-Salazar, Trujillo, Portocarrero-Aya and Whitehead2012; Paschoalini et al., Reference Paschoalini, Trujillo, Marmontel, Mosquera-Guerra, Paitach and Julião2021).
Data on the distribution and habitat use of aquatic mammals such as the Amazon River dolphin can be difficult to obtain, and this has been identified as a key knowledge gap for this group (Campbell et al., Reference Campbell, Alfaro-Shigueto, Aliaga-Rossel, Beasley, Briceño and Caballero2022). Many of these species are widely distributed, are highly mobile and can occur in areas that are difficult for researchers to access. The habitat use of river cetaceans has mostly been estimated visually through boat-based surveys (Gomez-Salazar et al., Reference Gomez-Salazar, Portocarrero-Aya, Trujillo, Caballero, Bolaños-Jimenez and Utreras2010, Reference Gomez-Salazar, Trujillo, Portocarrero-Aya and Whitehead2012; Pavanato et al., Reference Pavanato, Gomez-Salazar, Trujillo, Lima, Paschoalini, Ristau and Marmontel2019; Aliaga-Rossel & Duran, Reference Aliaga-Rossel and Duran2020; Aliaga-Rossel & Escobar-ww, Reference Aliaga-Rossel and Escobar-ww2020) and through mark–recapture via photographic identification (McGuire & Henningsen, Reference McGuire and Henningsen2007; Gómez-Salazar et al., Reference Gómez-Salazar, Whitehead, Trujillo, Whitehead, Gomez-Salazar and Trujillo2011). Acoustic surveys, in which hydrophones or acoustic data loggers record echolocation clicks and whistles emitted by the cetaceans, have also been used in freshwater settings. They have been able to provide a relative measure of abundance, and elaborate diel patterns and habitat use under less favourable weather conditions and when cetaceans are submerged (Tregenza et al., Reference Tregenza, Martin and da Silva2007; Dong et al., Reference Dong, Wang, Wang, Li, Mei and Wang2015; Campbell et al., Reference Campbell, Alfaro-Shigueto, Godley and Mangel2017). More recently, drones have been successfully used to study the spatial distribution of these species (Fürstenau Oliveira et al., Reference Fürstenau Oliveira, Georgiadis, Campello, Brandão and Ciuti2017; Oliveira-da-Costa et al., Reference Oliveira-da-Costa, Marmontel, Da-Rosa, Coelho, Wich, Mosquera-Guerra and Trujillo2020).
The use of satellite transmitters to track focal individuals is another method of generating information on the habitat use of these species. Satellite tracking can provide detailed information on individual animal movements and habitat use, as well as combining temporal and spatial information at the broader regional and global scales (Gredzens et al., Reference Gredzens, Marsh, Fuentes, Limpus, Shimada and Hamann2014; Sveegaard et al., Reference Sveegaard, Galatius, Dietz, Kyhn, Koblitz and Amundin2015). Satellite transmitters have been used successfully to track river dolphins but examples of this approach are limited (Mosquera-Guerra et al., Reference Mosquera-Guerra, Trujillo, Oliveira-da-Costa, Marmontel, Van Damme and Franco2021, Reference Mosquera-Guerra, Trujillo, Pérez-Torres, Mantilla-Meluk, Franco and Valderrama2022). In addition to providing information on dispersal and migration movements of these species, tracking can provide information on their behaviour, their relationship with environmental conditions and how these govern their distribution, and the importance of these environments to their life history (Davis et al., Reference Davis, David, Meÿer, Sekiguchi, Best, Dassis and Rodríguez2014; Gredzens et al., Reference Gredzens, Marsh, Fuentes, Limpus, Shimada and Hamann2014). Data from telemetry can also be used to identify overlaps with negative anthropogenic effects or impacts (Queiroz et al., Reference Queiroz, Humphries, Mucientes, Hammerschlag, Lima and Scales2016; Hart et al., Reference Hart, Iverson, Fujisaki, Lamont, Bucklin and Shaver2018; Frankish et al., Reference Frankish, Cunningham, Manica, Clay, Prince and Phillips2021). This information is critical for identifying high-priority habitats and developing conservation measures such as the designation and regulation of protected areas or the development of policies to mitigate anthropogenic threats (Graham et al., Reference Graham, Witt, Castellanos, Remolina, Maxwell, Godley and Hawkes2012; Le Corre et al., Reference Le Corre, Jaeger, Pinet, Kappes, Weimerskirch and Catry2012; Scott et al., Reference Scott, Hodgson, Witt, Coyne, Adnyana and Blumenthal2012; Hays et al., Reference Hays, Bailey, Bograd, Bowen, Campagna and Carmichael2019).
The Amazon River dolphin is facing increasing anthropogenic threats, including from fisheries, which leads to competition for resources, bycatch and intentional killing (Campbell et al., Reference Campbell, Mangel, Alfaro-Shigueto, Mena, Thurstan and Godley2020). The species' habitat has been degraded by mining, logging and agricultural conversion (Gomez-Salazar et al., Reference Gomez-Salazar, Trujillo, Portocarrero-Aya and Whitehead2012). The construction of dams, mainly in Brazil, is an expanding threat, with 175 dams operating or under construction in the Amazon basin, as well as at least 428 more planned over the next 30 years (Forsberg et al., Reference Forsberg, Melack, Dunne, Barthem, Goulding and Paiva2017; Anderson et al., Reference Anderson, Jenkins, Heilpern, Maldonado-ocampo, Carvajal-vallejos and Encalada2018; Almeida et al., Reference Almeida, Hamilton, Rosi, Barros, Doria and Flecker2020).
Additionally, the Amazon Waterway has been approved and is under contract for construction. This proposed Waterway involves dredging sites across four main rivers of the Amazon basin and the expansion of ports to facilitate ship navigation across the Amazon, Ucayali and Marañón Rivers (InfrAmazonia, 2022). The goal is to create a network that facilitates transportation within the Peruvian Amazon but also to and from Brazil by opening an outlet to the Atlantic for Peruvian trade to the north and to the Pacific for Brazil. The total population of the Amazon River dolphin is unknown and it is categorized as Endangered on the IUCN Red List (da Silva et al., Reference da Silva, Trujillo, Martin, Zerbini, Crespo, Aliaga-Rossel and Reeves2018). Knowledge of the ecology of the Amazon River dolphin, particularly its reliance on the diverse habitats available, is critical for improving the conservation prospects for this species.
Here we expand on our previous analysis of Amazon River dolphin movements (Mosquera-Guerra et al., Reference Mosquera-Guerra, Trujillo, Oliveira-da-Costa, Marmontel, Van Damme and Franco2021) to focus on the Peruvian Amazon. We estimate core areas and home ranges and put this information into the context of habitat disturbance from anthropogenic activities and the conservation status of and prospects for this species in the Peruvian Amazon.
Study areas
We undertook tag deployment in August 2018, before a period of rising waters (October–May), in two areas in the north-eastern Peruvian Amazon (Fig. 1). The first location was in the Pacaya Samiria National Reserve, in the Yanayacu–Pucate River, close to the confluence of the Marañón and Ucayali Rivers in Loreto State. Pacaya Samiria National Reserve covers an area of 20,800 km2 and is a Ramsar site with a relatively abundant population of Amazon River dolphins (Gomez-Salazar et al., Reference Gomez-Salazar, Trujillo, Portocarrero-Aya and Whitehead2012). The second location was Nucuray, Loreto, a tributary of the Marañón River, close to the confluence of the Marañón and Huallaga Rivers.

Fig. 1 The study area in north-east Peru, showing the distribution of the Amazon River dolphin Inia geoffrensis (da Silva et al., Reference da Silva, Trujillo, Martin, Zerbini, Crespo, Aliaga-Rossel and Reeves2018), Pacaya Samiria Natural Reserve, and the two sites where the satellite tags were deployed (in the Yanayacu–Pucate River, close to the confluence of the Marañón and Ucayali Rivers in Pacaya Samiria National Reserve, and in Nucuray, a tributary of the Marañón River, close to the confluence of the Marañón and Huallaga Rivers.
Methods
Tag deployment
We captured dolphins using the purse seine method (Plate 1; Read & Westgate, Reference Read and Westgate1997). Only eight adults and subadults were selected for tagging, based on body length, following previous descriptions (Martin & da Silva, Reference Martin and da Silva2018). We used SPOT-299A tags (Wildlife Computers, Redmond, USA), measuring 21 × 31 cm and weighing 70 g in air. We programmed the tags to turn off during low satellite pass coverage (i.e. during 4.00–7.00 and 16.00–20.00 GMT) and to transmit up to 250 locations per day. We attached the transmitters to the proximal part of the dorsal fin according to the methodology of Wells et al. (Reference Wells, Schwacke, Rowles, Balmer, Zolman and Speakman2017) using a 6 mm cordless drill and a 1.5 mm polyoxymethylene bolt with silicone tubing. The attachment installation process took 17–20 min and we kept the dolphins moist using sponges and covered their eyes to reduce disturbance. Veterinarians were present to monitor the dolphins’ health.

Plate 1 Installation of satellite tags on Amazon River dolphins Inia geoffrensis. (a) An Amazon River dolphin is caught with a local purse seine-type net called a boliche. (b) The dolphin is transferred to the shore with help of the boat and net. (c) The individual is transported to the beach. (d) The dolphin is placed on a stretcher and the tag is installed within 20 min. (e) The dolphin is released with the satellite transmitter attached. Photos: M. Pajuelo.
Filtering data
We received locations via the ARGOS data collection and location system (ARGOS, 2023). We removed near-duplicate positions, defined as positions that occurred ≤ 2 min after a position fix from the same individual (Ropert-Coudert et al., Reference Ropert-Coudert, Van de Putte, Reisinger, Bornemann, Charrassin and Costa2020; March et al., Reference March, Metcalfe, Tintoré and Godley2021). We then filtered the data using a speed, distance and angle filter that removed all location class Z values and points with unrealistic swimming speeds (> 3 m/s; da Silva, Reference da Silva, Perrin, Würsig and Thewissen2008) or improbable turning angles (we removed locations creating angles < 15° or < 25° if the lengths of the vectors were greater than 2.5 or 5.0 km, respectively) using the argosfilter package (Freitas et al., Reference Freitas, Kovacs, Lydersen and Ims2008) in R 4.2.0 (R Core Team, 2021).
Post-processing of location data
Many of the satellite locations were outside the main river channel because of the error inherent in ARGOS locations. We therefore created a river mask using OpenStreetMap (2020) and reallocated each position to the closest river cell (100 m resolution) within the error ellipses from the ARGOS Kalman Filter. Common speed filters, such as that used for the pre-filtering of the data, consider a Euclidean space and do not account for displacement across river boundaries. The two closest points could be separated by larger distances if they were located in different tributaries. Therefore, we calculated the speed between consecutive locations along the river path using the gdistance package in R and estimated the maximum speed threshold by calculating the 95% quantile (vmax; van Etten, Reference van Etten2017). We removed a location if the speed from a previous location or to a subsequent location exceeded vmax. We conducted this filtering approach twice. After filtering, we then interpolated the tracks at regular intervals (i.e. 2 h) along the river. We broke up tracks with data gaps in excess of 7 days, for separate regularization (i.e. we did not interpolate within gaps). We chose the location on the river using the ARGOS location error ellipses. We depicted the ellipse with the river mask for each location, and from multiple possible locations on the river we selected the one closest to the Argos location centroid.
Home range estimates
We applied dynamic Brownian bridge movement models to estimate the utilization distribution (UD, i.e. the variation in intensity of use over space expressed as a bivariate probability density function; Marzluff et al. Reference Marzluff, Knick, Millspaugh, Millspaugh and Marzluff2001) for all of our tracking data using the move package (Kranstauber et al., Reference Kranstauber, Smolla and Scharf2021) in R. Unlike standard utilization distribution estimators (e.g. minimum convex polygon and kernel density estimation), the dynamic Brownian bridge movement model estimates home range by considering the temporal and behavioural features of movement trajectories (Kranstauber et al., Reference Kranstauber, Kays, Lapoint, Wikelski, Safi and Kranstauber2012). In our model we specified a location error of 2 km, and to estimate the variance of Brownian motion (σ 2 m), we used a moving window size of 11 locations and a margin of 29 locations to account for potential differences in behavioural movement patterns. We selected these parameters by trial and error, determining which parameters resulted in home and core ranges that best matched the location points, considering the speed and spatial restrictions the species has as well as the raster resolution. Estimates were then clipped with the raster layer of the Amazon River developed using OpenStreetMap features with a resolution of 200 m. Values for the 50% and 95% UDs were calculated from the polygon contours extracted from the dynamic Brownian bridge movement model for core use and home ranges, respectively.
Spatial threat overlap
We gathered spatial data layers to examine the scope of three proximal anthropogenic threats that are of types considered the most significant for river cetaceans globally (Pavanato et al., Reference Pavanato, Melo-Santos, Lima, Portocarrero-Aya, Paschoalini and Mosquera2016; Brownell et al., Reference Brownell, Reeves, Thomas, Smith and Ryan2017, Reference Brownell, Reeves, Read, Smith, Thomas and Ralls2019; Campbell et al., Reference Campbell, Alfaro-Shigueto, Aliaga-Rossel, Beasley, Briceño and Caballero2022): dams, the Amazon Waterway and fisheries. The temporal span of the threat layers varies, and therefore we included layers that do not overlap completely with the tracking periods. We sourced dam data from Anderson et al. (Reference Anderson, Jenkins, Heilpern, Maldonado-ocampo, Carvajal-vallejos and Encalada2018), including dams that are extant, under construction or proposed. We obtained information on the location of dredging and port sites on the Amazon Waterway from InfrAmazonia (2022), and calculated the distance from the point of each dolphin's home range that was closest to the nearest dredging site and existing and proposed dams along the river network. For fisheries we used catch data (kg) from the government fisheries agency DIREPRO-Loreto (DIREPRO-L, Reference DIREPRO-L2016; Wildlife Conservation Society, 2020b), which are available by fishing zone (there are 927 fishing zones in 29 rivers/tributaries in Loreto State). We used this information as a representation of regions with varying fishing pressures because of the inherent error in data collection and analysis from self-reported fishing effort and landings. We utilized the same classification categories as the original sources, ranging from the lowest (75–50,000 kg) to the highest extraction rates (2,500,000–7,867,282 kg) during the 4-year period 2016–2019. We calculated the per cent of the dolphin's home range area (95% UD) in each category of fishery catch during 2016–2019 and per cent of overlap with the Pacaya Samiria National Reserve.
Results
We analysed locations for eight Amazon River dolphins (one female, seven males) with a body length of 170–197 cm and a weight of 64–112 kg (Table 1). The mean number of satellite locations per individual was 802 ± SD 434. Tags provided data for a mean of 88 days (Table 1). The mean minimum distance travelled per day was 3.5 ± SD 0.5 km, the longest by an individual was 8.5 km and the shortest was 2.3 km. The maximum displacement from the deployment site averaged 31 ± SD 16 km (Table 1, Fig. 2). There was no significant correlation between maximum displacement and overall duration of tracking period (r(6) = 0.14, P = 0.7).
Table 1 Summary of Amazon River dolphin Inia geoffrensis tag deployments in the north-eastern Peruvian Amazon (Fig. 1), grouped by deployment site and date. The table shows the duration of the tracking period, total number of detections, utilization distribution (UD) estimates (95% UD home range, and 50% UD core range; Fig. 2) and maximum displacement (furthest point from the deployment site) for each individual, and the mean, standard deviation and range for all tagged individuals.

1 F, female; M, male.
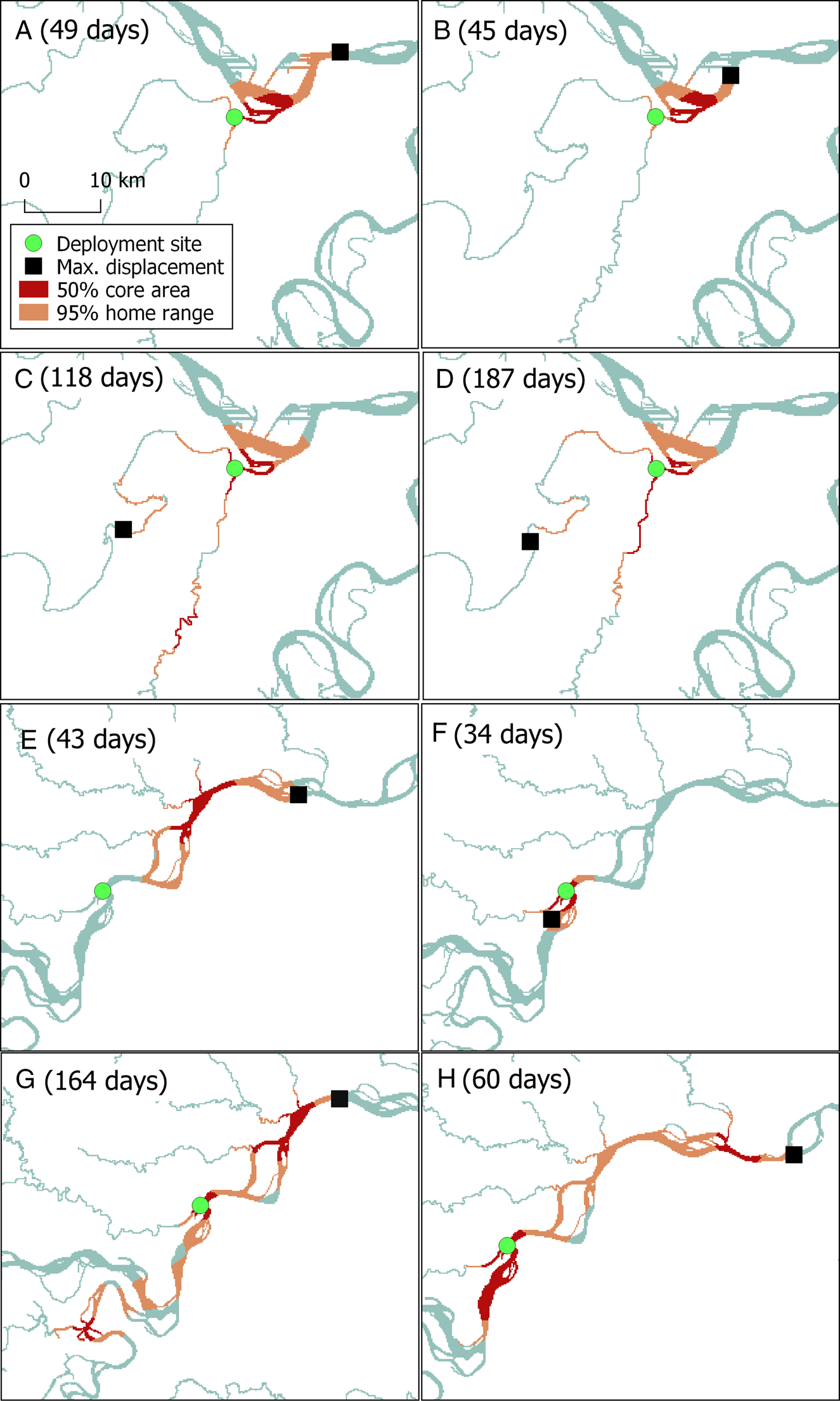
Fig. 2 Estimates of the core and home range areas of eight Amazon River dolphins tagged in north-eastern Peru (individuals A–D in Pacaya Samiria National Reserve, and individuals E–H in Nucuray; Fig. 1, Table 1), with total duration of tracking in parentheses, and the sites where the tags were deployed and the maximum displacement of each dolphin from the deployment site.
Home range analysis showed that the tagged dolphins moved in tributaries (Yanayacu–Pucate, Nucuray), main rivers (Marañón) and confluences (of Marañón and Huallaga Rivers, and Marañón and Amazon Rivers; Fig. 2). The mean home range area (95% UD) was 53.9 ± SD 28.9 km2. When averaged per deployment site, dolphins from Pacaya Samiria National Reserve had a smaller mean home range than those from Nucuray, the unprotected area, with home ranges of 38.3 ± SD 5.6 km2 and 69.4 ± SD 33.9 km2, respectively (Table 1, Fig. 2). The mean core area (50% UD) was 16.7 ± SD 9.7 km2. Dolphins tagged in the unprotected area had a core area of 22.5 ± SD 10.9 km2, whereas in the Reserve the mean core area was 10.9 ± SD 2.0 km2. Home range sizes had a positive but non-significant correlation with the duration of the tracking period (r(6) = 0.25, P = 0.55).
Dolphins tagged in the protected area had a mean overlap of 51% with Pacaya Samiria National Reserve. Those that were tagged in Nucuray had a minimal overlap with the Reserve, with a mean value of 11%.
All satellite-tracked dolphins moved within areas where there are small-scale fisheries (Figs 3a & 4), including overlap with low (range 20–84%), medium (range 15–49%) and high (range 1–30%) levels of fishery extraction. Dolphins tagged in the protected area had the greatest overlap with fishery extraction. Mean distance to the nearest existing dam was 444 ± SD 112 km, and mean distance to the nearest proposed dam was 252 ± SD 94 km (Fig. 3b, Table 2). Dolphins were close to two proposed Waterway dredging sites: a mean of 90 ± SD 48 km from Puinaha and 162 ± SD 84 km from Progreso (Table 2, Fig. 3b).
Table 2 Minimum distance (nearest point from 95% UD; Table 1) to dams and proposed dredging sites for the Amazon Waterway for each of the eight monitored dolphins and per cent overlap of home ranges with protected areas.

1 E, existing dam; P, proposed dam.

Fig. 3 The spatial extents in north-eastern Peru of (a) small-scale fishing catches (in kg) and (b) locations of extant, under construction and proposed dams, ports and dredging sites of the Amazon Waterway project, with corrected dolphin locations from ARGOS and the species' distribution (da Silva et al., Reference da Silva, Trujillo, Martin, Zerbini, Crespo, Aliaga-Rossel and Reeves2018). (Readers of the printed journal are referred to the online article for a colour version of this figure.)

Fig. 4 Overlap of the home ranges (95% utilization distribution; see text for details) of the eight monitored Amazon River dolphins (A–D; Table 1) with fisheries catch. Dolphins A–D were tagged in Pacaya Samiria National Reserve and E–H outside the Reserve. The grey bars indicate either a lack of fishing activity or the absence of fisheries data. (Readers of the printed journal are referred to the online article for a colour version of this figure.)
The home ranges of dolphins from both sites were a similar mean distance to the closest proposed dam (252.0 ± SD 95.7 km at Pacaya Samiria National Reserve and 252.0 ± SD 92.1 km at Nucuray) and dredging site (126.5 ± SD 79.2 km at Pacaya Samiria National Reserve and 125.3 ± SD 75.7 km at Nucuray).
Discussion
Home range results
Our results indicate that Amazon River dolphins have variable home range sizes that can cover > 100 km2. As many of the individuals in our sample were subadults, these movements could be a proxy for the movements of adult females (Martin & da Silva, Reference Martin and da Silva2006). These home range sizes were similar to the findings of previous studies of Inia species. Using a kernel density estimator, Mosquera et al. (Reference Mosquera-Guerra, Trujillo, Oliveira-da-Costa, Marmontel, Van Damme and Franco2021) estimated a mean home range of 59 km2 (range 6–233 km2) for 23 Amazon River dolphins, and using VHF radio transmitters, Martin & da Silva (Reference Martin and da Silva1998) estimated the distances covered by 53 Amazon River dolphins in Mamirauá Sustainable Development Reserve, Brazil, recording maximum displacements from their deployment location of up to 225 km (Martin & da Silva, Reference Martin and da Silva1998; Martin et al., Reference Martin, da Silva and Salmon2004).
Individuals displayed differences in the magnitude of movement at each of the two sites; dolphins tagged in the Reserve had smaller home ranges than those tagged in Nucuray. Although eight dolphins is a small sample, we can hypothesize about the potential factors that could contribute to differences in ranging behaviour. A potential cause is that a higher prey density in the Reserve means dolphins have to travel less far to search for food and therefore their home ranges are smaller. The diet of Amazon River dolphins is diverse: they consume at least 43 species of fish from 19 families (da Silva, Reference da Silva1983). The stomach of an individual Inia contained the remains of 15 fish with a mean length of c. 65 mm (McGuire & Winemiller, Reference McGuire and Winemiller1998). A high density of dolphins has been shown to correlate with a high density of characin fishes in main rivers (Mintzer et al., Reference Mintzer, Lorenzen, Frazer, da Silva and Martin2016), and prey availability could be one of the principal reasons for the elective movement of dolphins between suitable habitats (Martin & da Silva, Reference Martin and da Silva2004a). Confluences are also areas with high dolphin density as fish aggregate in these nutrient-rich areas (Gomez-Salazar et al., Reference Gomez-Salazar, Trujillo, Portocarrero-Aya and Whitehead2012; Pivari et al., Reference Pivari, Pagliani, Lemos, Lima and Gravena2021).
Another possible factor driving differences in home range sizes between protected and unprotected areas is habitat availability. We found that the monitored dolphins moved in tributaries, confluences and main rivers. Previous research based on boat surveys found that Amazon River dolphins exhibit differing habitat use between the low- and high-water seasons, with a preference for main rivers when the water level is low and for tributaries and flooded forests when water levels are at their highest (Martin et al., Reference Martin, da Silva and Salmon2004; Gomez-Salazar et al., Reference Gomez-Salazar, Trujillo, Portocarrero-Aya and Whitehead2012). It is possible that dolphins tagged in the unprotected area swam to the main river to forage for larger prey but preferred the Nucuray tributary for refuge and rest (Martin & da Silva, Reference Martin and da Silva2004b), explaining their larger home ranges. In contrast, dolphins in Pacaya Samiria National Reserve did not need to travel far to access areas that allowed all of these activities. These matters warrant further study with an increased sample size.
Limitations
Our analysis was constrained by a few key factors. We tagged only one female but found her home range size was within that of males from the same site. Any potential effects of sex on home range size are not yet well known. Previous studies have shown that males are more common in major river habitats than females and that females prefer habitats inside flooded forests (Martin & da Silva, Reference Martin and da Silva2004b). In addition, mark–recapture studies have revealed that male Amazon River dolphins exhibit similar levels of site fidelity to that of females despite their preference for large river systems (Martin et al., Reference Martin, da Silva and Salmon2004). We recommend that future satellite telemetry studies give priority to monitoring similar numbers of males and females.
Another limitation of our study was that it was not designed to look at seasonal changes in movement patterns. Our tags lasted for a mean of 88 days, similar to the 107 day mean of Mosquera et al. (Reference Mosquera-Guerra, Trujillo, Oliveira-da-Costa, Marmontel, Van Damme and Franco2021). We tagged all individuals whilst water levels were rising, and therefore we recommend that further monitoring be conducted when water levels are low to see whether and how dolphin movement changes under these circumstances. We also recommend that studies use tag models with batteries that last up to 1 year or tag deployments that are timed to facilitate year-round monitoring. These could be coupled with non-invasive methods such as environmental DNA or passive acoustic monitoring to better monitor movement patterns. Water-level fluctuation also affects the delineation of river spatial boundaries, having a potential impact on the river mask used in our analysis. Freshwater research and management could benefit from improved river maps. Developing robust, open-access data infrastructures containing such information for rivers has been identified as a priority for freshwater conservation (Maasri et al., Reference Maasri, Jähnig, Adamescu, Adrian, Baigun and Baird2022).
Implications for conservation
The overlap of home ranges with anthropogenic threats highlights patterns that could help to identify priority areas for the conservation of river dolphins in Peru. The home ranges of all monitored dolphins overlapped with fishing grounds. Although fishing catch does not necessarily correlate with fishing effort, we used it as an indicator of fishing pressure. A previous study found a positive correlation between effort (mean 433 kg landings per trip, 12 fishing trips per year) and landings in the Loreto fisheries (Tello & Bayley, Reference Tello and Bayley2001). The commercial fishing fleet of Loreto primarily uses purse seine-type nets and gillnets and, to a lesser degree, drag nets (Tello-Martin & Montreuil-Frias, Reference Tello-Martin and Montreuil-Frias1994; Alvarez Gómez & Rios Torres, Reference Alvarez Gómez and Rios Torres2009). We therefore hypothesize that in areas with medium and high fishing catch, dolphins are more likely to be taken as bycatch. Bycatch is an understudied threat to river cetaceans because of the lack of comprehensive impact assessments and the absence of consolidated data (Raby et al., Reference Raby, Colotelo, Blouin-Demers and Cooke2011; Whitty, Reference Whitty2016; Dewhurst-Richman et al., Reference Dewhurst-Richman, Jones, Northridge, Ahmed, Brook and Freeman2020; Campbell et al., Reference Campbell, Alfaro-Shigueto, Aliaga-Rossel, Beasley, Briceño and Caballero2022). Previous research in Peru has documented cetacean bycatch incidents in purse seine-type nets and gillnets (locally called honderas and agalleras, respectively; Campbell et al., Reference Campbell, Mangel, Alfaro-Shigueto, Mena, Thurstan and Godley2020), but given the proximity of our tagged dolphins to fisheries, even in protected areas, the overlap could be higher than reported previously. Pacaya Samiria National Reserve allows managed fishing by local communities, which is sustainable in areas close to small communities (Kirkland et al., Reference Kirkland, Eisenberg, Bicerra, Bodmer, Mayor and Axmacher2020). However, given the high overlap of the home ranges of dolphins tagged in the Reserve and fishery extraction, further research is needed to understand their exposure to risk. In priority areas for river dolphin conservation, onboard observer programmes should be implemented to estimate the frequency of bycatch and the fate of dolphins taken. In addition, overfishing could also potentially be a threat to Amazon River dolphins (e.g. by depleting fish prey; Allan et al., Reference Allan, Abell, Hogan, Revenga, Taylor, Welcomme and Winemiller2005), although there has been only limited research on this (Campbell et al., Reference Campbell, Alfaro-Shigueto, Aliaga-Rossel, Beasley, Briceño and Caballero2022).
Compared to river dolphin populations in Brazil (Araújo & Wang, Reference Araújo and Wang2015; Pavanato et al., Reference Pavanato, Melo-Santos, Lima, Portocarrero-Aya, Paschoalini and Mosquera2016), those in Peru are distant from extant dams. However, all proposed dams in Peru are upstream of and close to dolphin populations, and are therefore likely to cause alterations to key dolphin habitats. Downstream effects of dams on river dolphins have been observed and should be considered in Peru before construction begins. In Brazil, Araguaian dolphin densities were 68% lower downstream of the Tucuruí dam compared to upstream and dolphins shifted their range in response to dam construction (Paschoalini et al., Reference Paschoalini, Almeida, Trujillo, Melo-Santos, Marmontel and Pavanato2020). Similar effects have been observed in other river cetacean species, such as the Yangtze finless porpoise Neophocaena asiaeorientalis asiaeorientalis after the construction of the Three Gorges and Gezhouba dams, which caused flow regime alterations and reductions in fish spawning (Wang, Reference Wang2009; Fang et al., Reference Fang, He, Han, Duan, Huang and Chen2014; Chen et al., Reference Chen, Zhang, Wang, Liu, Wan and Yu2017).
Dredging could have negative effects on the Amazon River dolphin population similar to those that have occurred in Yangtze finless porpoise populations, where dredging has been correlated with higher cortisol and lower testosterone levels (Nabi et al., Reference Nabi, Hao, McLaughlin and Wang2018). Entrainment (i.e. the process by which surface sediment is incorporated into a fluid flow), habitat degradation, noise, contaminant remobilization and sedimentation can all affect benthic communities, which in turn can affect cetaceans indirectly via prey displacement (Todd et al., Reference Todd, Todd, Gardiner, Morrin, MacPherson, DiMarzio and Thomsen2015). Noise also affects dolphins directly as it often causes communication masking (when the ability to detect or recognize a sound of interest is reduced by the presence of another sound; Erbe et al., Reference Erbe, Reichmuth, Cunningham, Lucke and Dooling2016). Construction of the Amazon Waterway is currently under contract to commence but has been delayed because of objections from Indigenous and local communities and environmental groups, who claim that the impact assessments were not scientifically robust and the project is not viable in the long term (Sierra Praeli, Reference Sierra Praeli2020). Following construction of the Waterway, dredging will be undertaken regularly to maintain navigability. Greater vessel traffic in these areas could lead to collisions with dolphins and an increase in underwater noise.
These effects of water infrastructure projects and interactions with fisheries are cumulative with other threats such as climate change, pollution and hunting for use as bait (Palmer et al., Reference Palmer, Reidy Liermann, Nilsson, Flörke, Alcamo, Lake and Bond2008; Mintzer et al., Reference Mintzer, Martin, da Silva, Barbour, Lorenzen and Frazer2013; Barbosa et al., Reference Barbosa, Carvalho, Gravena, de Almeida, Mussy and Sousa2021). Although the Amazon River dolphin population is believed to be larger than that of other threatened river dolphin species, the total population size is unknown; however, it is known to be decreasing (Martin & da Silva, Reference Martin and da Silva2022). We therefore recommend a precautionary management approach. Peru is in a unique position as many of the drivers of habitat degradation we have described here are still at the proposal stage. On a broader scale, the Peruvian Amazon fisheries sector requires a stronger legislative framework that recognizes the central role of subsistence fishing in food security in the region and considers the realities of fishing by remote riverine communities (Wildlife Conservation Society, 2020a). This would be an important first step towards better organizing fisheries to then promote more sustainable fishing gear (e.g. hooks, harpoons) and estimate bycatch rates of river dolphins. In the case of dams and the Amazon Waterway, governments must consider explicitly the potential effects that dams will have at the basin scale and on freshwater species, as they will affect not only aquatic mammals but also the fisheries sector and food security (Winemiller et al., Reference Winemiller, McIntyre, Castello, Fluet-Chouinard, Giarrizzo and Nam2016). Without careful consideration, new dams will inevitably cause a decline in biodiversity and ecosystem services.
Acknowledgements
We thank the South American River Dolphin Initiative and WWF–Peru for funding this study; M. Pajuelo, N. Acuna, F. Nishio, P. Colchao and the fishers and community members who participated in tag deployment; and the editor and an anonymous reviewer for their comments. Elizabeth Campbell has a doctoral fellowship from WWF–Education for Nature.
Author contributions
Fieldwork: EC, JAS, JCM, JLM; data analysis, writing, revision: all authors.
Conflicts of interest
None.
Ethical standards
Satellite tagging was conducted under national research permits (RD 515-2018 PRODUCE, RJ 003-2018SERNANP) and was reviewed and approved by the University of Exeter Ethics Committee (eCORN001707), and this research otherwise abided by the Oryx guidelines on ethical standards.









