Introduction
The volcano rabbit Romerolagus diazi is endemic to central Mexico, restricted to the Ajusco–Chichinautzin mountains and the Popocatépetl, Iztaccíhuatl, Pelado and Tláloc volcanoes. Known locally as the teporingo or zacatuche, the species is distributed over c. 386 km2 (Velázquez et al., Reference Velázquez, Romero, León, Velázquez, Romero and López Paniagua1996) at elevations of 2,800–4,250 m (Fa & Bell, Reference Fa, Bell, Chapman and Flux1990). The volcano rabbit is considered a habitat specialist and depends on wild subalpine bunchgrass communities (known locally as zacatonales) characterized mainly by Muhlenbergia spp., Festuca spp. and Jarava ichu and associated with trees of the genera Pinus, Alnus and Quercus (Hoth et al., Reference Hoth, Velázquez, Romero, Leon, Aranda and Bell1987; Velázquez, Reference Velázquez1993). The species’ habitat has been destroyed gradually for agriculture, ranching and logging and by forest fires (Portales et al., Reference Portales, Reyes, Rangel, Velázquez, Miller, Ellis and Smith1997). Populations of the volcano rabbit are now at risk and this species is categorized as Endangered, both by the Mexican government (SEMARNAT, 2010) and on the IUCN Red List (AMCELA et al., Reference Romero Malpica, Rangel Cordero, de Grammont and Cuarón2008).
The habitat of a species can be examined at various scales, ranging from the local microscale (microhabitat) to the regional and global macroscales (Partridge, Reference Partridge, Krebs and Devie1978; Delcourt & Delcourt, Reference Delcourt and Delcourt1988; Dunning et al., Reference Dunning, Danielson and Pullian1992; Morrison et al., Reference Morrison, Marcot and Mannan2006). Heterogeneous landscapes are a common feature in the geographical distribution of most species of mammals, even those with a restricted distribution (Feldhammer et al., Reference Feldhammer, Drickamer, Vessey and Merritt2007). This mosaic of environmental conditions or habitat types is necessary to meet all of the requirements for species survival (Hansson, Reference Hansson1979). At the local scale habitat use can be studied by measuring the population density or abundance of focal species, assuming that the abundance of animals is higher in habitats that are more suitable for sustaining populations (Partridge, Reference Partridge, Krebs and Devie1978; Duncan et al., Reference Duncan, Colhoun and Foran1997). This suggests a unimodal distribution of population density along a gradient of habitat types (Velázquez, Reference Velázquez1993).
Although the current status of wild populations of R. diazi is critical, until now no information has been reported on their size, structure or demography, and knowledge of the ecological relationships between this species and its habitat is scant. The available information on habitat use is limited to the landscape scale (Velázquez, Reference Velázquez1993; Velázquez & Heil, Reference Velázquez and Heil1996). The only study carried out on the local scale was limited to the area of the Pelado volcano (Fa et al., Reference Fa, Romero and López-Paniagua1992). Therefore little is known about the habitat relationships of this species at local scales or about its peripheral populations (Velázquez et al., Reference Velázquez, Romero, León, Velázquez, Romero and López Paniagua1996).
Here we evaluate habitat characteristics and their relationship to the abundance of the volcano rabbit, and generate a habitat quality index based on this relationship. We counted rabbit latrines as an indirect method of determining population abundance (Palomares, Reference Palomares2001).
Study area
We investigated populations of the volcano rabbit in the Sierra Chichinautzin mountain range (Fig. 1). This is an important area because the populations here may be effectively isolated from other populations of the species that inhabit the Pelado, Tláloc, Popocatépetl and Iztaccíhuatl volcanoes, which are considered to be its core distribution areas (Velázquez et al., Reference Velázquez, Romero, León, Velázquez, Romero and López Paniagua1996). Part of the study area includes a protected area, the Chichinautzin Biological Corridor, which is located in the north-west of the state of Morelos, on the southern edge of the Federal District (Mexico City).

Fig. 1 Map of the Sierra Chichinautzin mountain range, with the locations of the 115 sampling sites. The rectangle on the inset shows the location of the main map in Mexico.
Methods
Using 1:20,000 aerial photographs from 1995 (National Institute of Statistics, Geography and Informatics, INEGI), we selected 115 sampling points at random above 2,800 m altitude (using contour lines at the 1:50,000 scale). We excluded urban areas (Tres Marías, Huitzilac, Coajomulco, Fierro del Toro and Parres). We visited all the sampling points to document habitat availability based on the presence of the grasses Muhlenbergia spp. and J. ichu. Sixty-four points had suitable habitat for R. diazi and 51 points did not (i.e. sites with fir, oak and pine forest, secondary vegetation, and deforested areas used mainly for growing oats and potatoes).
During June–December 2008 we estimated the abundance of the volcano rabbit at the sampling sites by counting the number of latrines. Following the method of Velázquez (Reference Velázquez1994) a group of 30 or more pellets was defined as a latrine. This method is considered reliable for estimating the population abundance of lagomorphs (Palomares, Reference Palomares2001) and has been used to estimate the population abundance of the volcano rabbit elsewhere in its range (Velázquez, Reference Velázquez1994). At each of the 64 sampling sites we surveyed one 50 × 50 m quadrat (0.25 ha) for fresh latrines.
We selected at random 22 of the 64 sampling points to describe the structure of the volcano rabbit's habitat. We measured seven vegetation attributes: tree height, cover, and diameter at breast height; shrub height and cover; and grass height and cover. We calculated standard deviation for grass height, shrub height and tree height, and for diameter at breast height as a proxy for habitat complexity. We used a clinometer to measure tree height and a telescopic rod to measure shrub height, in metres to the nearest centimetre. To calculate tree and shrub cover we measured the crown diameter of all trees and shrubs in the quadrat, using an electronic distance measuring tool. From the diameter the circular area of cover was calculated for each tree and shrub and these values were used to calculate percentage cover. We measured the height of 10 individuals of the two most abundant species of bunchgrass (Muhlenbergia macroura and J. ichu) and the percentage cover was estimated visually by the same person for each quadrat, thereby minimizing inter-observer variation.
The variables used to describe a habitat are typically interdependent and cannot be analysed individually (Cooley & Lohnes, Reference Cooley and Lohnes1971). Therefore we conducted a principal components analysis, which generates new axes from the linear combination of the original variables. The resulting principal components have the advantage of being orthogonal and are considered independent variables. For this analysis we used a correlation matrix because the habitat description variables differed in unit and scale (James & McCulloch, Reference James and McCulloch1990). We selected the principal components with the highest eigenvalues, the sum of which accounted for 90% of the variance. We used the principal components as new descriptive variables of the habitat to construct a habitat quality index. We formulated a set of 21 predictive models using different combinations of the major components, which we analysed using generalized linear models under a Poisson distribution. The independent variables were the principal components and the dependent variable was the abundance of the volcano rabbit (as indicated by the number of latrines recorded at each site). To select the best models we used the Akaike information criterion (Akaike, Reference Akaike, Petrov and Csaki1973). We ran the principal components analysis in Statistica v. 6.0 (StatSoft, Tulsa, USA) and the habitat analyses in R v. 2.14.1 and BiodiversityR (R Development Core Team, 2012; Kindt & Coe, Reference Kindt and Coe2005).
Results
The total area sampled was 160,000 m2. In the 64 quadrats found to have suitable habitat we counted 3,330 latrines. Twenty-seven (42%) of the sites had 1–50 latrines, 12 (19%) had 51–150 latrines and nine (14%) had 151–300 latrines. No latrines were found at 16 (25%) sites. The number of latrines varied across the altitudinal range of 2,760–3,760 m. Sites with 1–50 latrines were more common at 2,960–3,050 m and sites with 151–300 latrines were found at 3,060–3,150 m (Fig. 2).
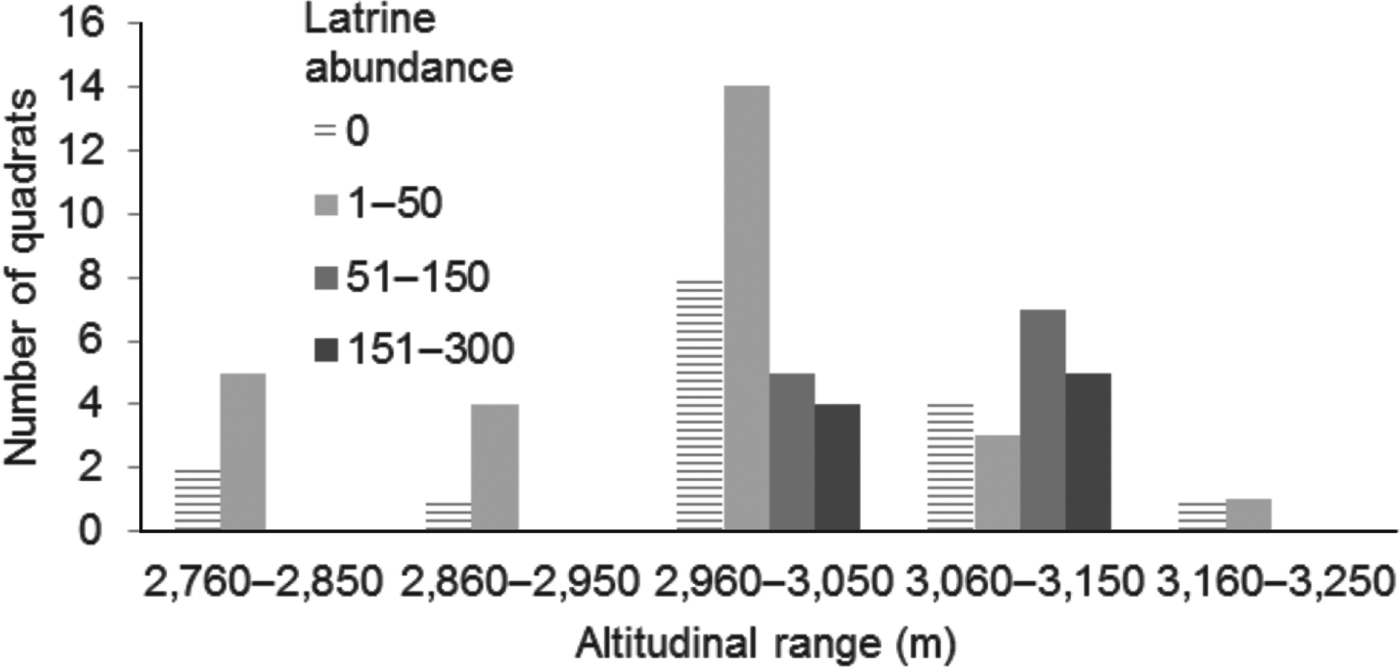
Fig. 2 Frequency distribution of latrine abundance with altitudinal range.
Of the 22 sites selected for habitat characterization, eight (36%) had 1–50 latrines, six (27%) had 51–150 latrines, five (23%) had 151–300 latrines and three (14%) had no latrines.
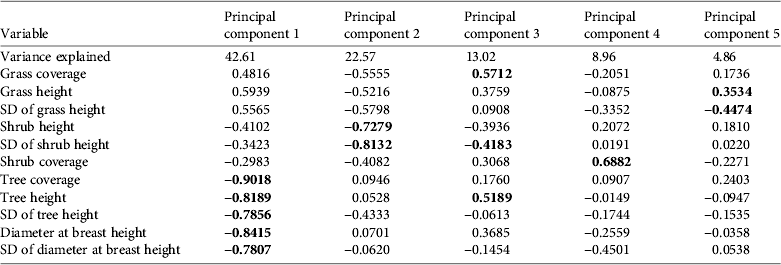
We retained five principal components that together accounted for 90% of the total variance in the habitat data (Table 1). The first component (PC1) was negatively associated with tree layer (cover, diameter at breast height, and height). PC2 was negatively associated with shrub height and standard deviation of the shrub height. PC3 was associated with a mixture of variables from different strata: it was positively associated with grass height and tree height and negatively associated with the standard deviation of the height of tall shrubs. PC4 was positively associated with shrub coverage and PC5 was negatively associated with the standard deviation of grass height and positively associated with grass height.
Table 1 Weights of the habitat variables for each of the principal components and the percentage of the variance accounted for by each component. Significant correlations are in bold.
Twenty-six different models were generated from combinations of the five principal components. The model with the lowest value for the Akaike information criterion held two major components (PC3 and PC5; Table 2). This model represents the habitat quality index. Analysis of the correlations between the original variables and the principal components (Fig. 3) revealed that R. diazi tended to be more abundant at sites with taller grasses, a higher percentage of grass cover and taller trees, and where there was less variation in shrub and tree height.

Fig. 3 The relative weights (correlations) of habitat variables with the four principal components. GC, grass cover; GH, grass height; SDGH, standard deviation of grass height; SH, shrub height; SDSH, standard deviation of shrub height; SC, shrub cover; TC, tree cover; HT, tree height; SDHT, standard deviation of tree height; DBH, diameter at breast height; SDDBH, standard deviation of diameter at breast height.
Table 2 Parameters of the best model selected based on Akaike information criterion, with estimated value, standard error, Wald's statistic, and P value.

Discussion
Our findings regarding the influence of the vegetation structure at a small scale (0.25 ha) on the distribution and abundance of the volcano rabbit in the Sierra Chichinautzin mountains broaden the understanding of the ecological relationships between this species and its habitat. A previous study of the relationship between the occurrence of the volcano rabbit and its habitat (Velázquez & Heil, Reference Velázquez, Romero, León, Velázquez, Romero and López Paniagua1996) focused on the floristic composition of vegetation and found that greater rabbit abundance was recorded in Festuca tolucensis and Trisetum altijugum–Festuca tolucensis communities. Based on that analysis Velázquez et al. (Reference Velázquez, Romero, León, Velázquez, Romero and López Paniagua1996) further concluded that the volcano rabbit prefers a subalpine habitat. Studies of other animal species have indicated that it is the vegetation structure and landscape configuration rather than the floristic composition that determine patterns of habitat occupancy (Rotenberry, Reference Rotenberry1985; Rosenberg & McKelvey, Reference Rosenberg and McKelvey1999; Pardini et al., Reference Pardini, De Souza, Braga-Neto and Metzger2005).
The availability of resources for feeding, breeding and shelter is a determining factor in the spatial distribution of the volcano rabbit, its habitat use and the shape of its home range (Gibb, Reference Gibb1993). It has also been shown that an animal's home range changes in response to its environment and that this response is directly related to habitat quality and resource distribution (Gibb, Reference Gibb1993; Hulbert et al., Reference Hulbert, Glenn, Elston and Racey1996; Lombardi et al., Reference Lombardi, Fernández, Moreno and Villafuerte2003; Stott, Reference Stott2003; White et al., Reference White, Newton-Cross, Gray, Ashford, White and Saunders2003).
Our findings that the presence and abundance of R. diazi are positively correlated with the height and cover of bunchgrass agree with those of another recent study (Hunter & Cresswell, Reference Hunter and Cresswell2014). These habitat features are a source of protection and food for the volcano rabbit (Cervantes & Martínez, Reference Cervantes and Martinez1992). The cover provided by grasses and the presence of a shrub layer may offer the volcano rabbit the best refuge from potential predators, including the bobcat Lynx rufus, the coyote Canis latrans, the long-tailed weasel Mustela frenata, the red-tailed hawk Buteo jamaicensis, the great horned owl Bubo virginianus and the rattlesnake Crotalus triseriatus (Cervantes-Reza, Reference Cervantes-Reza1981).
The Sierra Chichinautzin may be the last core area of bunch grassland in central Mexico (Cabrera-García et al., Reference Cabrera-García, Velázquez and Escamilla2006). However, this habitat is being degraded and destroyed by agricultural expansion, timber extraction and fire. This habitat loss is evidenced by the fact that only 64 of the 115 sampling sites in our survey were found to have suitable habitat for volcano rabbits.
The association between volcano rabbit abundance and the habitat quality index, together with the limited availability of suitable habitat documented in our field survey, suggests that populations of volcano rabbit in the study area are at risk of extinction. The species is a habitat specialist and therefore does not have the option of moving to a different type of habitat.
The data presented here, along with those previously reported for the landscape scale and for other areas of the species’ range (Fa et al., Reference Fa, Romero and López-Paniagua1992; Velázquez, Reference Velázquez1993; Velázquez et al., Reference Velázquez, Romero, León, Velázquez, Romero and López Paniagua1996), can be used to inform strategies for the conservation of the volcano rabbit's habitat. The ongoing loss of suitable habitat for the species warrants the implementation of an environmental education programme and collaboration between academia, government and NGOs to ensure that the people of central Mexico receive benefits for conserving the habitat of this endemic, restricted-range species.
Acknowledgements
We thank the laboratory group at the Facultad de Ciencias Biológicas of the Universidad Autónoma del Estado de Morelos for helping with the field work, and Bianca Delfosse for improving the English. This study was partially funded by the Comisión Nacional de Áreas Naturales Protegidas. Areli Rizo-Aguilar received a graduate studies scholarship from CONACYT (44564).
Biographical sketches
Areli Rizo-Aguilar is interested in the conservation biology of vertebrates and has been monitoring populations and habitat of the volcano rabbit. José Antonio Guerrero is working on the molecular systematics of bats and rodents and is also interested in the conservation and management of the volcano rabbit and its habitat. Mircea Hidalgo-Mihart has worked on the conservation and management of tropical mammals, especially carnivores and rodents, in western and south-eastern Mexico since 1998. Alberto González-Romero has been monitoring mammal populations in the Mapimi Biosphere Reserve in Durango, Mexico, and has also been studying the endemic ground squirrel of Perote.







