Introduction
Loss of ecological interactions of key species is an increasing threat to ecosystem structure and function (Redford & Feinsinger, Reference Redford, Feinsinger, Reynolds, Mace, Redford and Robinson2001; Soule et al., Reference Soule, Estes, Berger and Martinez del Rio2003), especially given the current declines in biodiversity (Secretariat of the Convention on Biological Diversity, 2010) and ecosystem services (MEA, 2005). Loss of interactions inevitably follows extinction but may also be lost before a species becomes demographically extinct, a situation known as ecological extinction. This occurs when a species no longer interacts significantly with other species, often due to a large reduction in its abundance (Estes et al., Reference Estes, Duggins and Rathburn1989). Ecological extinction may have far-ranging consequences if a species has unique and critical ecosystem functions (Novaro et al., Reference Novaro, Funes and Walker2000), such as is the case with the white-lipped peccary Tayassu pecari (Cetartiodactyla, Tayassuidae).
The white-lipped peccary is a social ungulate that forms large and cohesive groups which range widely in Neotropical forests from Argentina to Mexico. The species’ highly frugivorous habit and group behaviour affects plant survival, recruitment and spatial distribution through seed predation, dispersal, trampling and rooting (Beck, Reference Beck, Forget, Lambert, Hulme and Vander Wall2005, Reference Beck2006; Desbiez et al., Reference Desbiez, Santos, Keuroghlian and Bodmer2009; Keuroghlian & Eaton, Reference Keuroghlian and Eaton2009). By creating and maintaining wallows, which are critical aquatic habitat for many species, the white-lipped peccary also functions as an ecosystem engineer (Beck et al., Reference Beck, Thebpanya and Filiaggi2010). This species is also important prey for large carnivores such as puma Puma concolor and jaguar Panthera onca (Sowls, Reference Sowls1997; Aranda, Reference Aranda, Medellin, Equihua, Chetkiewicz, Crawshaw, Rabinowitz and Redford2002) and is widely recognized for its considerable socio-economic importance to poor forest-dwelling peoples (Bodmer et al., Reference Bodmer, Sowls, Taber and Oliver1993; Sowls, Reference Sowls1997), providing meat, hides, cash income (Stearman, Reference Stearman, Redford and Padoch1992) and sport (Reyna-Hurtado et al., Reference Reyna-Hurtado, Naranjo, Chapman and Tanner2010).
The loss of the white-lipped peccary has been documented from extensive areas (March, Reference March and Oliver1993; Peres, Reference Peres1996; Fragoso, Reference Fragoso, Fang, Bodmer, Aquino and Valqui1997, Reference Fragoso1999, Reference Fragoso, Silvius, Bodmer and Fragoso2004; Cullen et al., Reference Cullen, Bodmer and Valladares-Padua2000; Reyna-Hurtado & Tanner, Reference Reyna-Hurtado and Tanner2007). The species faces multiple threats across its geographical range including wide-scale habitat destruction and degradation, commercial harvesting, unsustainable levels of subsistence hunting and zoonotic diseases (probably spread from domestic livestock; Fragoso, Reference Fragoso, Fang, Bodmer, Aquino and Valqui1997, Reference Fragoso, Silvius, Bodmer and Fragoso2004; Altrichter & Boaglio, Reference Altrichter and Boaglio2004; Herrera et al., Reference Herrera, Abreu, Keuroghlian, Freitas and Jansen2008; Freitas et al., Reference Freitas, Keuroghlian, Eaton, de Freitas, Figueiredo and Nakazato2010). The white-lipped peccary is highly vulnerable to overexploitation and habitat fragmentation because it requires large and ecologically intact areas to maintain viable populations (March, Reference March and Oliver1993; Keuroghlian & Eaton, Reference Keuroghlian and Eaton2008b), which is especially problematic given the expanding industrial–agriculture frontier and elongating road networks across the Neotropics. The species is listed on Appendix II of CITES and classified as Near Threatened on the IUCN Red List (Reyna-Hurtado et al, Reference Reyna-Hurtado, Taber, Altrichter, Fragoso, Keuroghlian and Beck2008).
The ecological importance of the white-lipped peccary, combined with its importance to human communities, its wide geographical range, diversity of habitats and national and cultural contexts, creates a broad set of rationales for conserving the species, analogous to other large-bodied herbivores such as bison Bison bison (Sanderson et al., Reference Sanderson, Redford, Weber, Aune, Baldes and Berger2008) and elephants Loxodonta africana (Alexander, Reference Alexander2000; Blake & Hedges, Reference Blake and Hedges2004). Conservation priorities for individual species need to be developed on a scale commensurate with that of the taxa being considered (Sanderson et al., Reference Sanderson, Redford, Vedder, Ward and Coppolillo2002a; IUCN/SSC, 2008). Planning only within protected areas is unlikely to be sufficient when populations span a variety of habitats and land-use types beyond protected area boundaries (Sowls, Reference Sowls1997; Woodroffe & Ginsberg, Reference Woodroffe and Ginsberg1998). Landscape-scale approaches have therefore been recommended for the conservation of such wide-ranging species (IUCN/SSC, 2008; Redford et al., Reference Redford, Amato, Baillie, Beldomenico, Bennett and Clumet2010).
A major problem confronting conservation of the white-lipped peccary is the lack of recent and reliable information on its ecology, biology and population status synthesized across its range. The last assessment was over 15 years ago (Oliver, Reference Oliver1993). Data are difficult to obtain because of the remoteness of much of the species’ distribution, the large home ranges of peccary populations, lack of technical and management capacity, and low levels of government and international support for long-term studies. As a result, it is unclear why the white-lipped peccary has recently disappeared from vast areas (March, Reference March and Oliver1993; Peres, Reference Peres1996; Fragoso, Reference Fragoso, Fang, Bodmer, Aquino and Valqui1997, Reference Fragoso, Silvius, Bodmer and Fragoso2004; Reyna-Hurtado, Reference Reyna-Hurtado2007).
We therefore carried out a range-wide status assessment, starting in 2005, followed by > 3 years of additional data acquisition and analyses. We employed the range-wide priority-setting methodology first described for jaguars (Sanderson et al., Reference Sanderson, Redford, Chetkiewicz, Medellin, Rabinowitz and Robinson2002b) and later applied to other species in Latin America, including the tapir Tapirus terrestris (Taber et al., Reference Taber, Chalukian, Altrichter, Minkowski, Lizárraga and Sanderson2008) and American crocodiles Crocodylus acutus (Thorbjarnarson et al., Reference Thorbjarnarson, Mazzotti, Sanderson, Buitrago, Lazcano and Minkowski2006), and recently adopted in part by the IUCN Species Survival Commission in its guidelines on species conservation planning (IUCN/SSC, 2008). This methodology explicitly addresses how the distribution of a species varies across national and ecological geographies, based on a synthesized assessment of status across the species’ historical range. It also enables quantification of range changes over time and assessment of unknown areas. Our objectives were to: (1) update historical (c. 1900) and current distribution maps for the white-lipped peccary, (2) estimate its status across its range in terms of both political and ecological geographies, (3) identify key conservation areas for conservation action and monitoring, (4) identify and rank threats, and (5) synthesize information for conservation planning and action at national and international scales.
Methods
Forty-one researchers from 15 countries (Appendix 1) provided original survey data and expert opinion in a workshop process (described below) and subsequent contributions (Taber et al., Reference Taber, Chalukian, Altrichter, Minkowski, Lizárraga and Sanderson2008). Researchers provided the following information: (1) areas for which they had information about the species’ presence and status (area of knowledge), (2) coordinates of localities where the presence or absence of the species had been documented during the last 20 years, (3) the current and historical distribution of the species within areas of knowledge, and (4) key areas for the conservation of the species (peccary conservation units; Table 1). Each locality was defined as a circle of 10-km radius (315 km2). Additional points were obtained from the literature and incorporated into the database. The researchers also provided information for each locality and for peccary conservation units, including hunting pressure, deforestation, non-timber resource extraction, peccary population size and trends, herd size, land tenure, land use and effectiveness of protection. Assessments for some of these categories were subjective but because these researchers have the best information available given their experience in each area we included such information in the analysis, making qualifications as appropriate.
Table 1 Definitions of geographical data types used in this study.
Of the potential geographies for analysing range information and constructing conservation priorities we used the political geography of nations and an ecologically-based approach of regional habitat types, modified from globally delineated ecoregions (Dinerstein et al., Reference Dinerstein, Olson, Graham, Webste, Primm and Bookbinder1995) and Latin American habitat units (Anon., 1995), based on the principle that it is important to ensure the long-term survival of ecologically distinct populations of the white-lipped peccary. We identified and mapped 32 eco-geographical regions within the species’ range, and six principal habitat types, by further grouping similar eco-geographical regions. Each eco-geographical region indicates the region where it is found and the predominant habitat (e.g. SE Amazon/Tropical Moist Lowland Forest).
The workshop, held on 3–9 April 2005 in Santa Cruz de la Sierra, Bolivia, brought together 25 researchers, selected to maximize geographical coverage. During the workshop we revised each data layer (area of knowledge, localities, historical and current distribution, and peccary conservation units) separately. Modifications were incorporated using georeferenced digital photographs.
Distribution polygons were classified as containing peccary populations with one of three long-term survival prospects: high (populations relatively stable, with long-term survival likely in a large portion of the polygon; polygons > 1,000 km2); medium (most populations decreasing with their viability threatened by landscape transformation and/or other human activities); low (populations small and isolated, for the most part persisting in scarce habitat fragments). We also estimated the area of each distribution polygon where the species was subject to various threats and then totalled this information across the whole range.
Peccary conservation units were defined as ‘an area currently known or believed to contain a resident population of peccaries large enough to be potentially self-sustaining over the next 100 years, or an area containing fewer peccaries currently but with adequate habitat and a stable and diverse food base, such that the population could increase if threats were alleviated’. We identified the most relevant factors affecting peccary survival and qualitatively assessed their intensity within each peccary conservation unit by classifying it using a Likert scale as high, medium, none, or no information available. Experts were asked to assess each threat in each peccary conservation unit in terms of its intensity and the breadth of its geographical distribution; these data were then summarized at the range-wide level. Peccary conservation units were weighted according to the following algorithm: peccary conservation unit size was considered most important (weight = 31), followed by habitat destruction (25), hunting (20), disease (10), habitat quality (6), connectivity (4), and lack of resources (4).
During the workshop, participants assigned each factor a relative weighting such that their sum equalled 100. The intensity previously assigned to each factor was multiplied by the weight of that factor, and the resulting number was used to estimate the relative conservation status of each peccary conservation unit (Taber et al., Reference Taber, Chalukian, Altrichter, Minkowski, Lizárraga and Sanderson2008). This provided a continuum of estimators for conservation status, from poor to excellent, representing the combination of the weight of diverse factors important for the species’ survival within peccary conservation units. We later grouped peccary conservation units into low, medium, and high conservation status.
Results
Area of knowledge and data points
A total of 936 localities containing 6,378 detection records of the white-lipped peccary for 1985–2005, in combination with researchers’ knowledge, were used to map the current distribution of the species. At the time of the workshop we did not have any peccary locality data for French Guiana, Panama and Surinam. Large ecological areas for which we lack status information are the lowland rainforest of the Chocó-Darién and Central America (79,692 km2, 12% of the combined area of both eco-geographical regions). Status information was also lacking for approximately half the Amazonian mangroves (14,670 km2, 56% of the eco-geographical region; Table 2).
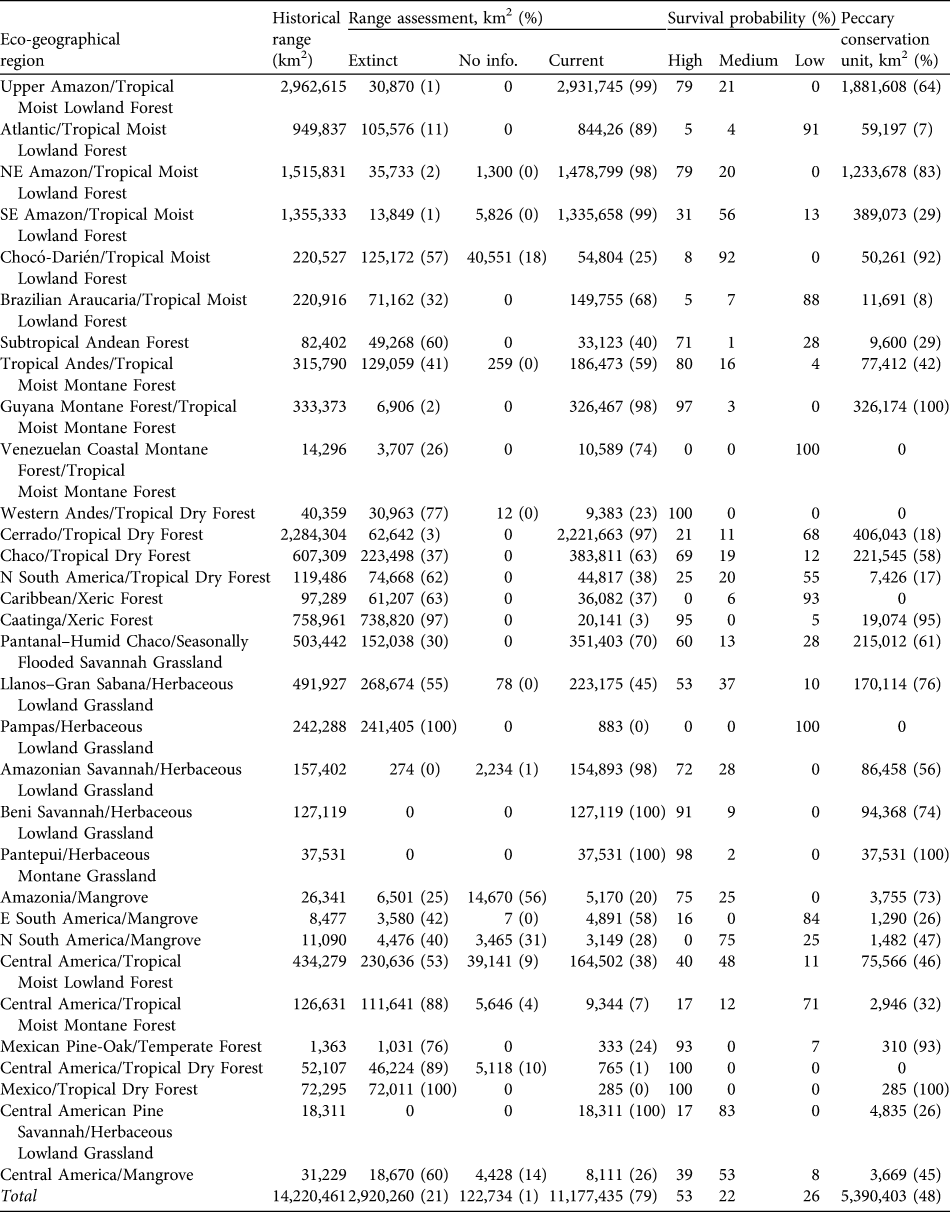
Table 2 For each eco-geographical region (see text for details) the area of historical range, area (and percentage) in which the white-lipped peccary Tayassu peccari is extinct, for which there is no status information and of the current range, percentage of current range in which the species is considered to have high, medium and low probabilities of long-term survival, and area (and percentage) of peccary conservation units.
Distribution and conservation status
We estimate that the historical range of the white-lipped peccary was 14,220,461 km2, encompassing 32 eco-geographical regions (Appendix 2; Table 2) in six principal habitat types (Appendix 3; Table 3). The current distribution extends over 11,177,435 km2 (79% of the historical range; Fig. 1; Table 2). The largest eco-geographical region polygons are in the upper Amazon, cerrado and north-east and south-east Amazon (Table 2). The largest principal habitat type is the Tropical and Subtropical Moist Broad-leaf Forest (Table 3), covering 62% of the current range. Brazil contains the largest range of the species (Table 4), comprising 66% of the total distribution. The largest percentage reductions in the species’ range have occurred in Central America, north-west South America, the arid regions of north-east Brazil and the fringe of the species’ southern range in Argentina and Brazil. In terms of total area lost, the largest reductions have occurred in the caatinga. The current range remains essentially intact in the Central American/Herbaceous Lowland Grassland, the Beni Savannah/Herbaceous Lowland Grassland, and the Pantepui/Herbaceous Montane Grassland eco-geographical regions (Table 2). In the principal habitat types the largest reduction has occurred in the Tropical and Subtropical Dry Forests, Savannahs and Shrublands and, in percentage terms, in the Mangroves (Table 3).
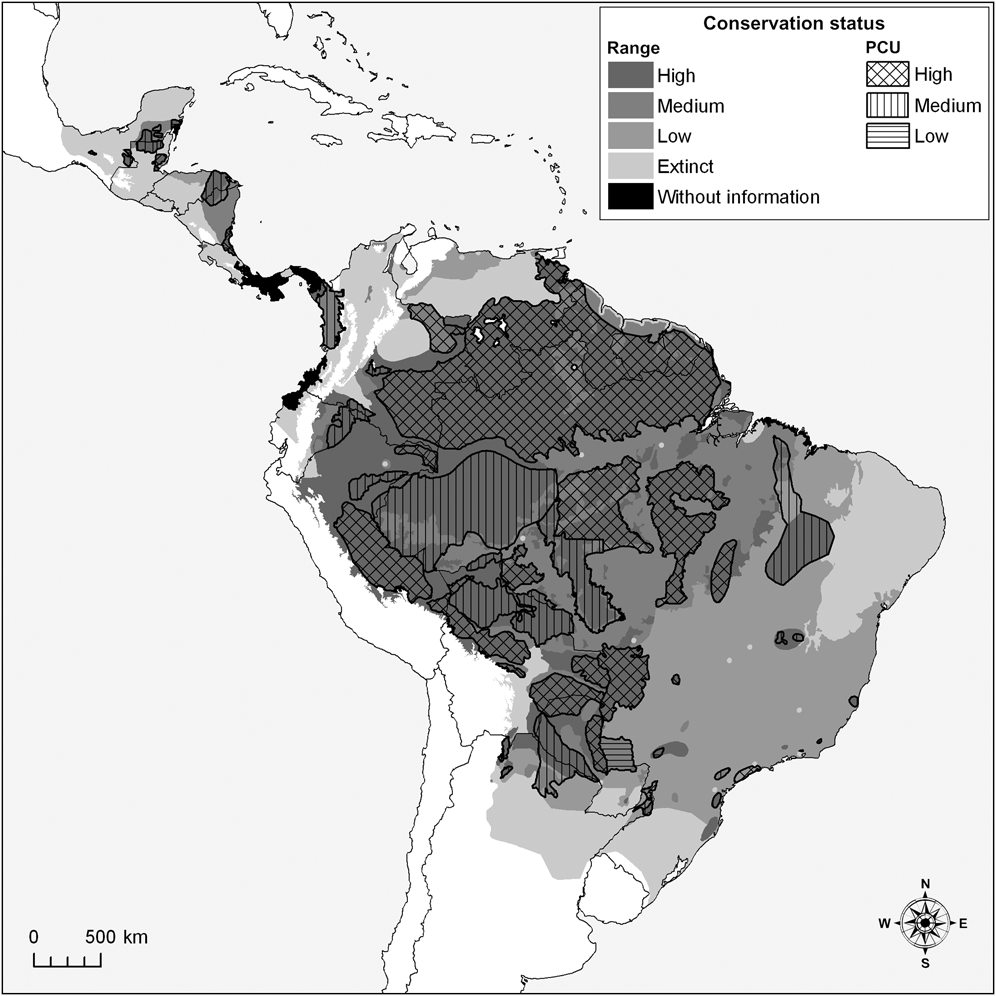
Fig. 1 Assessment of the white-lipped peccary Tayassu pecari range and conservation units (PCUs; see text for definition) as of high, medium or low conservation status (see text for details), and areas of the historical range where the species is now extinct or for which there is no information.
Table 3 For each principal habitat type (see text for details) the area of historical range, area (and percentage) in which the white-lipped peccary is extinct, for which there is no status information and of the current range, percentage of current range in which the species is considered to have high, medium and low probabilities of long-term survival, and area (and percentage) of peccary conservation units.
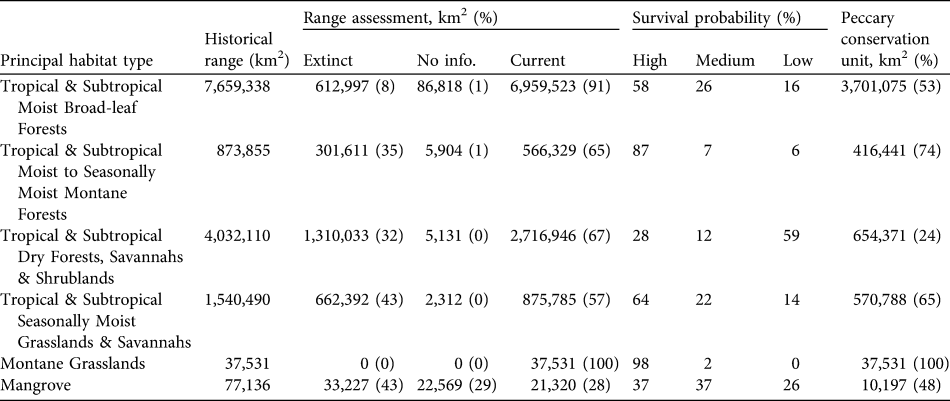
Table 4 For each range country the area of historical range, area (and percentage) in which the white-lipped peccary is extinct, for which there is no status information and of the current range, percentage of current range in which the species is considered to have high, medium and low probabilities of long-term survival, and area (and percentage) of peccary conservation units.
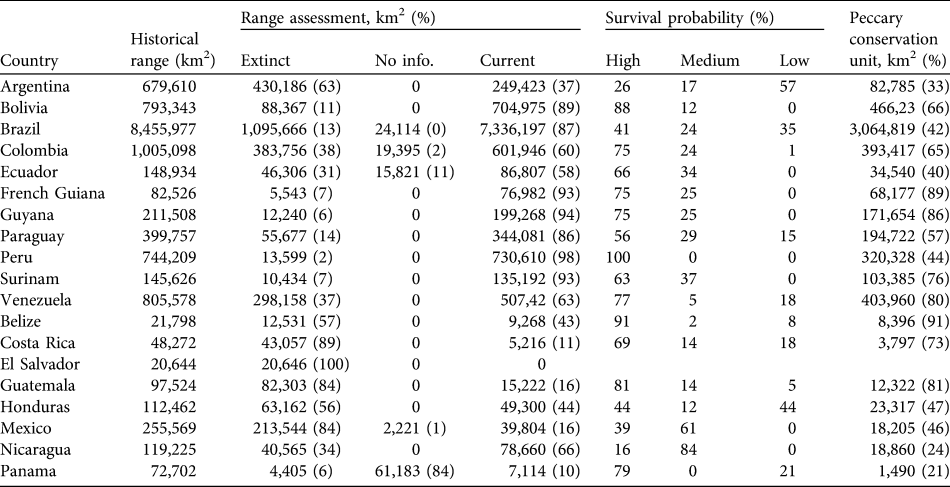
By country, the largest reduction in range area has occurred in Brazil, in 13% of the historical distribution of the species. The species has disappeared from all or a majority of its historical range in El Salvador (extinct), Costa Rica (89%), Guatemala and Mexico (84%) and Argentina (63%; Table 4).
The largest eco-geographical regions containing peccary populations with high probabilities of long-term survival are the Upper and North-east Amazon/Tropical Moist Lowland Forests (c. 79% of each region’s total area). The Cerrado/Tropical Dry Forest has the largest area containing small, fragmented, and isolated peccary populations with low survival probabilities. The Atlantic/Tropical Moist Lowland Forest also has large extensions containing populations with low survival probabilities (Fig. 1; Table 2).
The Tropical and Subtropical Moist Broad-leaf Forests is the principal habitat type with the largest absolute and percentage (53%) of its area considered to have peccary populations with high survival probabilities. The Tropical and Subtropical Dry Forests, Savannahs and Shrublands have the largest area comprising populations with low probabilities of survival (Table 3). Brazil has the largest area containing populations with high and medium probabilities of survival. However, 2.6 million km2 of the peccary distribution in Brazil contains populations with low survival probabilities. Peru and Bolivia have the largest percentage of the peccary distribution with high survival probabilities (100 and 88% respectively). Belize and Costa Rica each contain < 10,000 km2 with populations with high survival probabilities. In Nicaragua only 16% of the current peccary range contains populations with high survival probabilities.
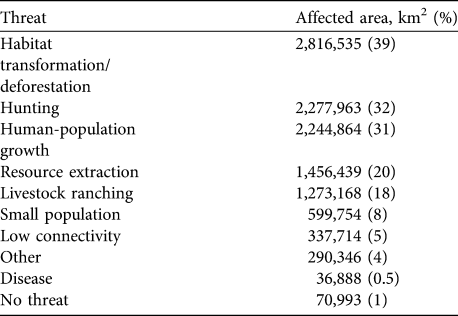
The two major threats to the white-lipped peccary are deforestation and habitat transformation, affecting 40% of its range. Hunting and human population growth have each affected c. 30% of the peccary distribution. Only 1% of the total range is considered to be unaffected by human activities (Table 5). These estimates reflect the area affected but not the intensity of the various threats.
Table 5 The main threats to the white-lipped peccary, with the area (and percentage) of the current range affected.
Peccary conservation units
We mapped 57 peccary conservation units covering 5,390,403 km2, (48% of the species’ current distribution; Table 6; Fig. 1). A larger number of units were classified as medium (n = 26) than as high (n = 19) or low (n = 8) conservation status but 65% of the total area assigned to the units was classified as being of high conservation status. The status of four small peccary conservation units could not be classified.
Table 6 Number (and percentage) and area (and percentage) of white-lipped peccary conservation units categorized by conservation status (see text for details).

Peccary conservation units varied in size from 119 km2 to > 2 million km2. The largest units are in central and northern South America and the smallest in Central America and south-eastern Brazil. The Upper and North-eastern Amazon/Tropical Moist Lowland Forests has almost 2 million and 1.3 million km2, respectively, designated as conservation units (Table 2). All other eco-geographical regions contained < 500,000 km2 in peccary conservation units. The greatest number of units, and the largest total area designated as units (68% of total), are in the Tropical and Subtropical Moist Broad-leaf Forests principal habitat type (Table 3). Brazil had the largest area designated as peccary conservation units (3,064,819 km2; Table 4), larger than all other units combined. Bolivia, Venezuela, and Colombia also have large areas designated as peccary conservation units (c. 400,000 km2 each), whereas Belize, Costa Rica, and Panama each have < 10,000 km2 of peccary conservation units.
Peccary herd sizes were estimated to be > 20 in 49% of the peccary conservation units. In about half of the total peccary conservation unit area the units were considered to contain populations of > 5,000. However, in 33% of units peccary population sizes were estimated to be 1,000–5,000 (Table 7). There was a relationship between peccary conservation unit size and the estimated peccary population: the mean peccary conservation unit size (248,769 km2) with > 5,000 peccaries was 100 times larger than the mean peccary conservation unit size with < 500 individuals. A similar relationship was observed between herd and peccary conservation unit sizes: the mean peccary conservation unit size (155,424 km2) with herds of > 20 individuals was 40 times larger than the mean peccary conservation unit size with smaller herds. Almost 70% of the total peccary conservation unit area was considered to contain stable peccary populations. However, only one peccary conservation unit (0.1% of the combined area of all peccary conservation units; Table 8) was considered to have a population that is increasing.
Table 7 Number, percentage, mean area and total area of peccary conservation units containing different herd and population sizes of white-lipped peccary.

Table 8 Number (and percentage) of peccary conservation units, and their area (and percentage) exhibiting increasing, decreasing or stable population trends or for which there is no information.

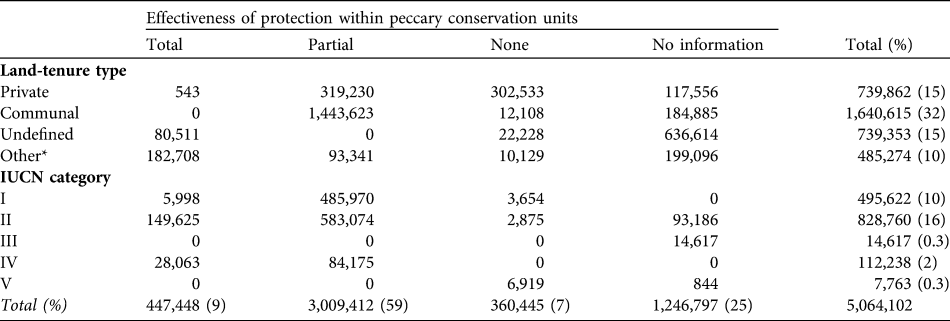
A larger number of peccary conservation units had medium rather than high or low levels of hunting (Table 9). Subsistence and opportunistic hunting pressure, respectively, were considered high in 28 and 16% of the units. Only two units were unaffected by subsistence hunting. Sport hunting was less prevalent. Approximately a third of the units had medium levels of deforestation pressure, whereas only 5% were unaffected by deforestation. Slightly more than half of the units (58%) had medium levels of extraction of natural resources (Table 10). More of the land area in peccary conservation units (32%) was under common property than other tenure regimes and c. 30% of the total peccary conservation unit area was protected as of 2005 under one of the IUCN (1994) protected area categories (Table 11). About 60% of the total peccary conservation units have partial protection, principally on common property lands. However, only 9% of the combined peccary conservation unit area was considered to provide effective protection despite the much higher percentage coverage in protected areas.
Table 9 Number (and percentage) of peccary conservation units under four types and intensity of hunting.
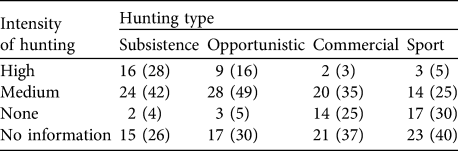
Table 10 The percentage of peccary conservation units under various deforestation and resource-extraction regimes

Table 11 Estimated area (km2) under different degrees of effectiveness of protection within peccary conservation units by various land-tenure types and IUCN (1994) categories.
* Mainly indigenous reserves and state lands
Discussion
Conservation status
Conservation of wide-ranging species such as the white-lipped peccary requires a broad geographical perspective. Our synthesis of observations across the range of this species (> 14,097,695 km2) shows that it has lost > 21% of its range since the beginning of the 20th century. Major range reductions have occurred in Central America, Mexico, north-west South America, the arid region of north-east Brazil and along the species’ southern limit in Argentina. The species is now extinct in El Salvador and its range has been reduced by > 60% in several other countries. Brazil, Peru and Bolivia are particularly important for this species; combined, they account for > 78% of the species’ current range.
White-lipped peccary populations in more xeric environments have been particularly affected. For example, 25% of the overall range loss has occurred in the Caatinga/Xeric Forest eco-geographical region of north-east Brazil, where the species persists in a fragmented area of < 20,000 km2. Other eco-geographical regions with poor prognoses for population survival are the Pampas/Herbaceous Lowland Grassland, where the peccary persist in < 900 km2, and the Cerrado/Tropical Dry Forest, where only 18% of the area is in peccary conservation units and most of the remaining area has populations rated with low and medium probabilities of survival. However, prospects for the species survival are better in other eco-geographical regions, including the Upper Amazon, which contains the largest contiguous range polygon and where this species is judged to have a medium to high probability of survival across the entire area.
Could the white-lipped peccary become ecologically extinct?
Given the vast range loss, representing local extinctions, and reductions in many of the remaining populations, we are concerned that this major ecosystem architect of the Neotropics could become ecologically extinct. Although 70% of the peccary conservation units are considered to have stable populations, only 1% of the species' range was assessed as completely free from anthropogenic threat (not considering effects of climate change such as changes in rainfall). Habitat loss and hunting accounts for threats in 70% of the species’ range, in agreement with previous studies (Fragoso, Reference Fragoso, Fang, Bodmer, Aquino and Valqui1997; Cullen et al., Reference Cullen, Bodmer and Valladares-Padua2000; Peres, Reference Peres2001; Altrichter & Boaglio, Reference Altrichter and Boaglio2004; Reyna-Hurtado, Reference Reyna-Hurtado2009; Reyna-Hurtado et. al, Reference Reyna-Hurtado, Rojas-Flores and Tanner2009). A serious problem is the low effectiveness of protection for this species across its range. Although partial protection exists in 60% of the combined area of all peccary conservation units, in only 9% of this area (the key strongholds for this species’ survival) does the white-lipped peccary appear to be adequately protected. Furthermore, although 30% of the total peccary conservation unit area is within IUCN protected area categories, in only 20% of this area is protection considered effective for the species. These findings underline the limitations of protected areas in Latin America for conserving large, wide-ranging species.
Any ecological extinction of the white-lipped peccary could have far reaching consequences given their role as ecological architects and ecosystem engineers in Neotropical forests (Beck, Reference Beck, Jorgensen and Fath2008; Keuroghlian & Eaton, Reference Keuroghlian and Eaton2009; Beck et al., Reference Beck, Thebpanya and Filiaggi2010). At high densities this species may have the highest total vertebrate species biomass, reaching up to 230 kg km-2 (Peres, Reference Peres1996; Silman et al., Reference Silman, Terborgh and Kiltie2003). Many ecosystem changes have been documented following local extinction of the white-lipped peccary (Silman et al., Reference Silman, Terborgh and Kiltie2003; Wyatt & Silman, Reference Wyatt and Silman2004). The species consumes > 144 plant species of 38 families, in addition to a variety of invertebrates, bird eggs, fishes, snakes, frogs and small mammals (Bodmer, Reference Bodmer1989; Altrichter et al., Reference Altrichter, Carrillo, Saenz and Fuller2001; Beck, Reference Beck, Forget, Lambert, Hulme and Vander Wall2005, Reference Beck2006; Keuroghlian & Eaton, Reference Keuroghlian and Eaton2008a; Desbiez et al., Reference Desbiez, Santos, Keuroghlian and Bodmer2009). It is an important seed predator and, together with tapirs, is the only species that can crush many hard nuts (Kiltie, Reference Kiltie1981, Reference Kiltie1982; Beck, Reference Beck, Forget, Lambert, Hulme and Vander Wall2005; Keuroghlian & Eaton, Reference Keuroghlian and Eaton2008a). It also disperses many small-seeded species (e.g. Ficus spp.; Altrichter et al., Reference Altrichter, Sáenz and Carrillo1999; Beck, Reference Beck, Forget, Lambert, Hulme and Vander Wall2005) and mycorrhizae spores, crucial to maintaining symbiotic relationships with many plant species (H. Beck, unpubl. data). Trampling by large groups of foraging white-lipped peccary can affect seedling densities (Asquith et al., Reference Asquith, Wright and Clauss1997; Roldan & Simonetti, Reference Roldan and Simonetti2001; Keuroghlian & Eaton, Reference Keuroghlian and Eaton2009). Given its high biomass and diverse consumption of fruit and seeds, this species may regulate populations of numerous terrestrial frugivores via exploitative and interference competition (Beck, Reference Beck, Forget, Lambert, Hulme and Vander Wall2005, Reference Beck2006; Keuroghlian & Eaton, Reference Keuroghlian and Eaton2008a). White-lipped peccary are also important prey for jaguars and pumas (Kiltie & Terborgh, Reference Kiltie and Terborgh1983; Aranda, Reference Aranda, Medellin, Equihua, Chetkiewicz, Crawshaw, Rabinowitz and Redford2002). Increased carnivore–livestock conflicts have been documented where white-lipped peccaries have disappeared, presumably because of prey-switching by carnivores (Azevedo & Conforti, Reference Azevedo and Conforti2008; Cavalcanti & Gese, Reference Cavalcanti and Gese2010).
However, we do not know how these numerous ecological interactions with other species and the abiotic environment change with variations in white-lipped peccary population density. Such information is required for a full examination of whether the white-lipped peccary is threatened with ecological extinction. The data and analyses presented here do, nevertheless, demonstrate major contractions in the species’ range, which suggest declines, or at least modifications, of the various ecological roles of the white-lipped peccary in places where the species is now absent.
Conservation of the white-lipped peccary
The size of peccary conservation units and the risk of deforestation and hunting appear to be critical factors for the survival of this species. White-lipped peccaries use habitats non-randomly, underlining their need for a variety of ecosystem types and their associated fruiting species (Keuroghlian & Eaton, Reference Keuroghlian and Eaton2008a; Lee & Peres, Reference Lee and Peres2008; Galetti et al., Reference Galetti, Giacomini, Buenos, Bernardo, Marques and Bovendorp2009; Keuroghlian & Eaton, Reference Keuroghlian and Eaton2009). Genetic studies in the Brazilian Pantanal have demonstrated that the white-lipped peccary must be managed at the scale of landscapes and metapopulations (Biondo et al., Reference Biondo, Keuroghlian and Miyaki2008). Recent data indicate a low degree of genetic differentiation between two populations 80 km apart (Biondo et al., Reference Biondo, Keuroghlian, Gongora and Miyaki2010). Individual groups also range over very large areas (from < 40 km2 to 120–200 km2; Fragoso, Reference Fragoso1998; Carrillo et al., Reference Carrillo, Saenz and Fuller2002; Reyna-Hurtado et al., Reference Reyna-Hurtado, Naranjo, Chapman and Tanner2010). Accordingly, conservation efforts need to focus on landscape conservation of large, continuous and ecologically intact areas containing a mosaic of habitat types, as defined by the peccary conservation units.
The range-wide assessment presented here will allow national-level conservation planners to appreciate the range of major habitat types utilized by the white-lipped peccary. Conservation action for this species, primarily habitat protection and improved management of hunting (including total prohibition in some places), and policy changes, are urgently required (Galetti et al., Reference Galetti, Giacomini, Buenos, Bernardo, Marques and Bovendorp2009). Our findings emphasize, in particular, the need to focus on the conservation of the ecologically distinct populations in dry forests, savannahs and shrublands.
We identified several regions where the distribution and conservation status of the white-lipped peccary is unknown. In addition, even though the major part of this species’ distribution is in the Tropical Moist Lowland Forest of the Upper Amazon and the adjoining Cerrado eco-geographical regions, status and ecological studies in these areas are sparse, with a density of detection locality points of < 1 per 23,000 km2 (Taber et al., Reference Taber, Chalukian, Altrichter, Minkowski, Lizárraga and Sanderson2008). These geographies should be research priorities in future studies.
Acknowledgements
This work was supported by a grant from the Gordon and Betty Moore Foundation to the Wildlife Conservation Society, which also provided logistic support. The latter’s Landscape Ecology and Geographic Analysis Program guided the data collection and geographical information system analyses. We thank Karen Minkowski from that programme. The Liz Claiborne and Art Ortenberg Foundation and the Wildlife Trust also provided financial and other support. We especially thank the 41 peccary specialists and other colleagues (Appendix 1) who shared their original data: such unswerving generosity is key for conservation.
Appendices
The appendices for this article are available online at http://journals.cambridge.org
Biographical sketches
Mariana Altrichter works to conserve peccaries and studies their importance in subsistence economies of northern Argentina and Costa Rica. Andrew Taber is a biologist working to reconcile trade-offs between conservation and development across the tropics. Harald Beck’s research focuses on non-trophic and trophic interactions of large mammal species and the resulting ecological consequences for the ecosystem. Rafael Reyna-Hurtado’s research focus is on movement ecology and conservation of threatened tropical ungulates and primates of southern Mexico. Leonidas Lizarraga is a geographical information system specialist studying spatial–temporal human and ecological relationships between protected areas and their surroundings. Alexine Keuroghlian’s research includes using white-lipped peccary ecology and genetic data to define ecological corridors in the Pantanal and its highland plateaus. Eric W. Sanderson is a landscape ecologist interested in developing sustainable relationships between people and nature at all scales.














