Introduction
Vast tracts of forested landscape formerly inhabited by the tiger Panthera tigris have now been lost to human habitation, causing a sharp decline in ungulate populations and confining many of the remaining tiger populations to small, isolated patches of forests (Smith et al., Reference Smith, Ahearn and McDougal1998). In India, despite 30 years of conservation efforts, an expanding human population has caused considerable reduction in the tiger's habitat and a decline in prey and tiger numbers (Seidensticker et al., Reference Seidensticker, Christie and Jackson1999). With the illegal killing of tigers for body parts accelerating the rate of local extinctions (Project Tiger, 2005), long-term conservation of the tiger requires the identification, protection and management of habitats to secure breeding populations as source pools and provide dispersal opportunities by linking habitat patches across the landscape (Johnsingh et al., Reference Johnsingh, Ramesh, Qureshi, David, Goyal and Rawat2004; Wikramanayake et al., Reference Wikramanayake, McKnight, Dinerstein, Joshi, Gurung and Smith2004).
The Terai-Arc Landscape, encompassing the Shivalik hills and the Terai flood plains parallel to the outer Himalayas, has been identified as an important landscape for the long-term conservation of the tiger in the Indian subcontinent (Wikramanayake et al., Reference Wikramanayake, McKnight, Dinerstein, Joshi, Gurung and Smith2004). With a human population density of > 500 people km-2 (the national average is 300 people km-2; Johnsingh et al., Reference Johnsingh, Ramesh, Qureshi, David, Goyal and Rawat2004), tiger populations occur in forest patches comprising a matrix of protected areas, multiple use forests (Forest Divisions), agricultural land and human habitation. The north-western portion of this landscape spans from the Sharda River bordering India and Nepal in the east to the Yamuna River in the west. This area, encompassing the protected areas of Corbett Tiger Reserve and Rajaji National Park, is fragmented into three disjunct units identified as Tiger Habitat Blocks, with poor or no connectivity because of anthropogenic disturbances (Johnsingh et al., Reference Johnsingh, Ramesh, Qureshi, David, Goyal and Rawat2004). Covering nearly 6,600 km2, this hilly (bhabar) tract could potentially support a minimum of 150 adult tigers if corridors were established and adequate protection provided (Johnsingh, Reference Johnsingh2006). From east to west these Tiger Habitat Blocks include multiple use forests covering c. 1,800 km2 from the west bank of the Sharda River to the east bank of the Gola River (Block III), multiple use forests, Corbett Tiger Reserve and the eastern part of Rajaji National Park covering c. 3,000 km2 from the west bank of the Gola River to the east bank of the Ganga River (Block II), and the western part of Rajaji National Park and multiple use forests covering c. 1,800 km2 from the west bank of the Ganga River to the Yamuna River (Block I), forming the north-western limit of tigers in the Indian subcontinent (Fig. 1). As populations at their range limits are more susceptible to local extinctions, ensuring the long-term persistence of the tiger within this landscape requires quantitative information on existing populations and opportunities for dispersal and connectivity between populations.

Fig. 1 North-western limit of the Terai-Arc Landscape of India showing major rivers (Yamuna, Ganga, Gola & Sharda), Tiger Habitat Blocks (Block I, II & III), habitat corridors (Chilla-Motichur and Rajaji-Corbett) and the protected areas of Rajaji National Park and Corbett Tiger Reserve.
Many areas of this landscape are inhabited by nomadic pastoralists, Gujjars. Although some of them are still nomadic (coming down to the bhabar during the winter and returning to high altitude pastures in the Himalayas during summer), most now reside permanently within the forests. Their presence in Rajaji National Park is mentioned as early as 1924 (Kumar, Reference Kumar1995). Until the formation of Rajaji National Park in 1983 permits were issued to the families within the Park to cut grass and lop branches for leaves to provide fodder for their livestock. As their livestock holdings (primarily buffaloes Bubalus bubalis) increased (from c. 4,000 to c. 20,000; Kumar, Reference Kumar1995), requirements for fodder increased proportionately. Intensive lopping, grazing and firewood extraction in these forests led to proliferation of invasive plant species and lack of regeneration of various tree species (Edgaonkar, Reference Edgaonkar1995). Following a voluntary pastoral Gujjar resettlement programme initiated by the Uttarakhand Forest Department in 2003, the Chilla range of east Rajaji National Park provided an opportunity to monitor changes in prey-predator populations. Subsequently, Harihar et al. (Reference Harihar, Pandav and Goyal2008) documented an increase in tiger density from 3 to 5 tigers per 100 km2 over 2004–2007. As the eastern Park forms the western limit of Tiger Habitat Block II, this recovery could be attributed to dispersing individuals from Corbett Tiger Reserve through the adjoining Lansdowne Forest Division. Additionally, evidence of breeding tigers has been documented since the initiation of the monitoring programme in 2004 (Harihar et al., Reference Harihar, Pandav and Goyal2008). It is therefore critical to assess the status of tigers and their prey in potential dispersal grounds west of the river Ganga (western Rajaji National Park). In this study we assessed the occurrence of tigers and leopards Panthera pardus based on sign encounter surveys along dry stream beds (raus), following which we estimated prey densities using line transects in conjunction with distance sampling methods and density of tigers using photographic capture-recapture sampling. We discuss our results in the context of the landscape and propose a potential recovery plan to ensure the long-term persistence of tigers in this area.
Study area
This study was conducted from December 2006 to April 2007 in six forest ranges (Chilla, Ghauri, Hardwar, Dholkhand east, Dholkhand west and Chillawali; Fig. 2) of Rajaji National Park, which is bisected by development activities such as the expansion of Haridwar and Rishikesh townships, establishment of Khand gaon settlements, Raiwala army camp, the Hindustan antibiotic factory and the 14 km long Rishikesh-Chilla power channel along the banks of the Ganga River. The c. 200 km2 eastern sector of the Park is the western limit of Tiger Habitat Block II (Fig. 1) and is connected with Corbett Tiger Reserve through Lansdowne Forest Division (the Rajaji-Corbett corridor; Johnsingh & Negi, Reference Johnsingh and Negi2003; Johnsingh et al., Reference Johnsingh, Ramesh, Qureshi, David, Goyal and Rawat2004). Whereas Gujjar settlements from within Chilla range have been resettled, the resettlement process is ongoing in Ghauri range. The c. 600 km2 western sector of the Park, which forms the eastern limit of Tiger Habitat Block I (Fig. 1), is narrowly connected to Tiger Habitat Block II through the highly disturbed Chilla-Motichur corridor (Johnsingh et al., Reference Johnsingh, Prasad and Goyal1990), and is contiguous with multiple use forests that extend westwards to Yamuna River. The Gujjar resettlement programme is still underway in some parts (Ramgargh, Chillawali and Dholkhand east), but settlements from the forest ranges of Hardwar, Dholkhand west, Motichur and Kansrau have moved out of the Park.
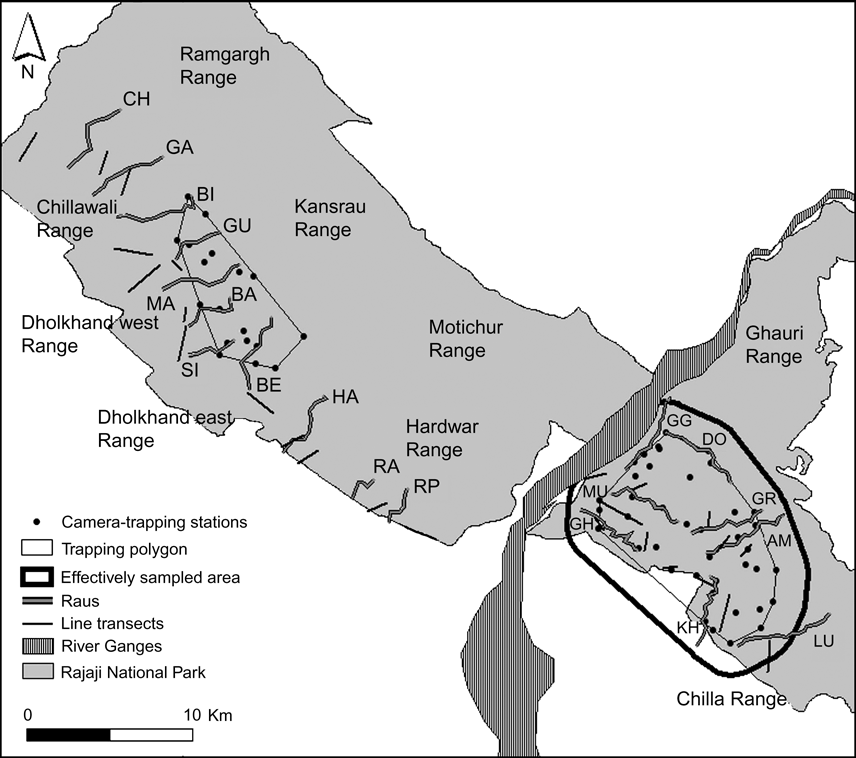
Fig. 2 Location of camera-trap stations (40 and 20, respectively, in the eastern and western sectors of Rajaji National Park; Fig. 1), trapping polygons, effectively sampled area used to estimate tiger density in the eastern sector of the Park, 24 permanent line transects used to estimate densities of wild prey, and raus (eight in the eastern and 11 in the western sector; for 2-lettered codes see Table 1) sampled for signs in Rajaji National Park from December 2006 to April 2007. Forest ranges are indicated.
Characterized by rugged hills over 400–1,000 m altitude, with steep southern slopes, and drained by seasonal rivers and streams running north to south, the forests of this region are categorized as Northern Indian Moist Deciduous Forest and Northern Tropical Dry Deciduous Forest (Champion & Seth, Reference Champion and Seth1968). With the valleys supporting extensive grasslands, the major vegetation associations are mixed forests comprising tree species such as Terminalia alata, Anogeissus latifolia, Lagerstroemia parviflora, Holoptelia integrifolia, Ehretia laevis, Aegle marmelos and sal Shorea robusta on the south facing slopes and sal dominated forests on the gentle north facing slopes. Apart from the tiger, leopards also occur in this area. The prey species of the tiger in the study area are sambar Cervus unicolor, chital Axis axis, barking deer Muntiacus muntjak, nilgai Boselaphus tragocamelus, wild pig Sus scrofa, goral Nemorhaedus goral, common langur Semnopithecus entellus, porcupine Hystrix indica, hare Lepus nigricollis and Indian peafowl Pavo cristatus. Domestic livestock (chiefly cattle and buffalo) ranging from the villages on the boundary of the forests are also potential prey species.
Methods
Sign surveys
Based on an intensive survey of the status of tigers in the three Tiger Habitat Blocks, Johnsingh et al. (Reference Johnsingh, Ramesh, Qureshi, David, Goyal and Rawat2004) suggested that transects on dry stream beds (rau) were ideal to record tracks and signs of large mammals. We therefore conducted sign surveys along 19 transects in raus (Fig. 2), totalling 80 km in December 2006. We surveyed 11 and eight raus, 2.75–4 km and 5 km in length, respectively, in the southern forest ranges of the western and eastern sectors (Fig. 2).
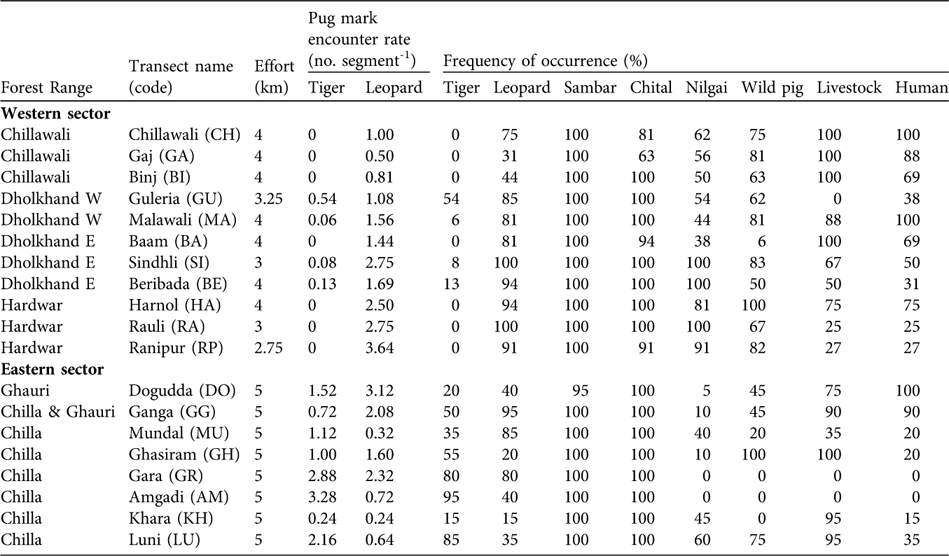
Surveys were carried out by teams of 2–4 biologists and assistants and took 3–4 hours (at the rate of 1.25–1.5 km h-1). Each transect was divided into 250 m segments. Indirect evidence of tigers and leopards (pug marks, scats and scrapes) were recorded. Length and breadth of pug marks were recorded to identify individuals and thus avoid double counts. From these data indices of relative abundance of the tiger and leopard were computed as pug mark encounter rate (number of distinct pug marks per 250 m segment). Signs (tracks and faecal remains) of principal prey species (sambar, chital, nilgai and wild pig) and anthropogenic disturbances (livestock, dog and human signs) were also recorded. Based on these data, frequency of occurrence of signs per segment (number of segments with signs/total number of segments surveyed), expressed as a percentage, was calculated. A carnivore index (frequency of occurrence of both tigers and leopards), prey index (the joint frequency of occurrence of all prey species) and disturbance index (frequency of occurrence of livestock and human signs) were computed for each transect and used as input variables in a hierarchical cluster analysis (Sneath & Sokal, Reference Sneath and Sokal1973). We used the single linkage (nearest neighbour) agglomerative clustering method with squared Euclidean distance (SPSS v. 14, SPSS, Chicago, USA) as a measure of dissimilarity to arrange the sampled raus into groups.
Estimation of prey density
Densities of wild prey were estimated using line transects and distance sampling (Anderson et al., Reference Anderson, Laake, Crain and Burnham1979; Burnham et al., Reference Burnham, Anderson and Laake1980; Buckland et al., Reference Buckland, Anderson, Burnham and Laake1993, Reference Buckland, Anderson, Burnham, Laake, Borchers and Thomas2001). A total of 24 permanent line transects were laid (12 each in the western and eastern sectors; Fig. 2), with lengths of 0.91–2.5 km and a total length of 33.5 km, covering all vegetation types. Each transect was walked three times by two observers over 06.15–09.30 from December 2006 to February 2007 (a total of 72 walks and 100.5 km). On every walk, species, group size, age-sex composition, sighting angle using a hand held sighting compass, and sighting distance measured by a laser range finder were recorded. Population density of principal prey species was estimated using the software Distance v. 5 Release 2 (Thomas et al., Reference Thomas, Laake, Strindberg, Marques, Buckland and Borchers2006). To model detection functions to estimate species' density the data for each species per transect were examined for signs of evasive movement and peaking at distances from the line of walk. Following this, the data were either truncated or reclassed so as to ensure a reliable fit of key functions and adjustment terms to the data. The Akaike Information Criterion (AIC) and goodness-of-fit tests were used to judge the fit of the model. Using the model thus selected, estimates of group density, group size and individual density were derived.
Estimation of tiger density
To estimate the population density of adult tigers we used photographic capture-recapture analysis (Karanth, Reference Karanth1995; Karanth & Nichols, Reference Karanth and Nichols1998). Trapping stations were selected based on the presence of secondary evidence such as pug marks, scats and scrapes that indicated the use of the area by tigers, therefore maximizing capture probabilities (Karanth, Reference Karanth1995). As we only had a total of 20 camera traps (STEALTHCAM IR1, Stealth Cam, Grand Prairie, USA), systematic sampling was carried out by trapping in spatially separated blocks in a phased manner. Each block consisted of 10 trap sites run for 15 consecutive days. Thus, each sampling occasion combined captures from 1 day drawn from each block. Fearing theft of the cameras during daytime, one trap-night was a 14-hour period (17.00–07.00) during which a pair of cameras was functional at each site. Using the network of roads each of the 10 trapping sites in a block, with an average inter-trap distance of 1.5 km, was checked on a daily basis and all photographs were downloaded to a laptop computer. Sampling in the eastern sector was carried out during January–February 2007 in 4 blocks with a total of 40 camera-trapping stations (Fig. 2), and trapping in the western sector was carried out during March–April 2007 in two sampling blocks with a total of 20 camera-trapping stations (Fig. 2). As the trapping grids in the eastern and western sectors were separated by c. 20 km, the resultant data sets were analysed separately.
Every tiger captured was given a unique identification number after examining the stripe pattern on the flanks, limbs and forequarters (Schaller, Reference Schaller1967; McDougal, Reference McDougal1977; Karanth, Reference Karanth1995). Following the identification of tigers, capture histories to estimate the tiger population were developed. Within the sampling period we tested for population closure using the closure test of the software CAPTURE (Otis et al., Reference Otis, Burnham, White and Anderson1978, White et al., Reference White, Anderson, Burnham and Otis1982; Rexstad & Burnham, Reference Rexstad and Burnham1991). The density (![]() ) of tigers in the study area was estimated as the population size (
) of tigers in the study area was estimated as the population size (![]() ) divided by the effective sampled area (A(Ŵ)), where A(Ŵ) was estimated by creating a polygon over the trapping stations (A) and adding a buffer width (Ŵ) estimated as half the mean maximum distance moved (½ MMDM) by recaptured tigers to the camera trap polygon (A; Karanth & Nichols, Reference Karanth and Nichols1998).
) divided by the effective sampled area (A(Ŵ)), where A(Ŵ) was estimated by creating a polygon over the trapping stations (A) and adding a buffer width (Ŵ) estimated as half the mean maximum distance moved (½ MMDM) by recaptured tigers to the camera trap polygon (A; Karanth & Nichols, Reference Karanth and Nichols1998).
Results
Occurrence patterns of tigers and leopards
We encountered pug marks of both the tiger and leopard (Table 1). The mean pug mark encounter rate for leopards was 1.61 ± SE 0.23 km-1, with no significant difference between eastern (1.38 ± SE 0.37) and western sectors (1.71 ± SE 0.29; Mann-Whitney U test, Z = -0.99, P = 0.322). The mean pug mark encounter rate for tigers was 0.72 ± SE 0.23 km-1, with a significant difference between eastern (1.61 ± SE 0.37) and western sectors (0.07 ± SE 0.04; Mann-Whitney U test, Z = -3.64, P = 0.001). Although we detected tiger signs in all of the eight sampled raus in the eastern sector (pug mark encounter rates of 0.24–3.28 per 250 m; Table 1), we detected tiger signs in only four of the 11 sampled raus in the western sector (pug mark encounter rates ranging of 0.06–0.54 per 250 m; Table 1). Signs of prey species (sambar, chital, nilgai and wild pig) were found across the Park (Table 1). We detected evidence of livestock and people in all sampled raus except two (Table 1).
Table 1 The 19 transects (in raus) surveyed (a total effort of 80 km) in December 2006 in the western and eastern sectors of Rajaji National Park (Figs 1–2), with survey effort, encounter rate of tiger and leopard pug marks per 250 m segment, and frequency of occurrence (number of 250 m segments with signs/total number of segments surveyed, as a percentage) of signs of tiger and leopard, of the four main ungulate tiger prey species (sambar, chital, nilgai and wild pig) and of livestock and human activity.
The hierarchical cluster analysis based on carnivore index, prey index and disturbance index produced two distinct clusters (Fig. 3). One comprised the raus in the eastern sector and the other those of the western sector. Among the raus in the western sector, two further classes, primarily dominated by patterns of human occupancy were identified (Gujjar evacuated areas and Gujjar occupied areas). Human occupancy thus potentially influences movements of large mammals and, in particular, sites used by tigers.
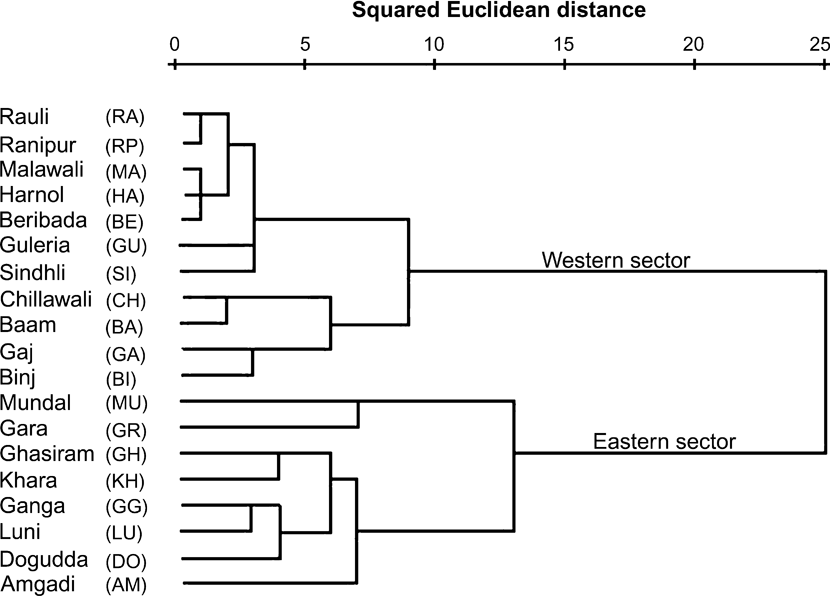
Fig. 3 A hierarchical cluster analysis (see text for further details) using carnivore index, prey index and disturbance index for 19 sampled stream beds (raus) in Rajaji National Park (Fig. 2, Table 1) during December 2006. The raus separate into two distinct clusters of the 11 and eight raus in the western and eastern sectors, respectively.
Prey densities
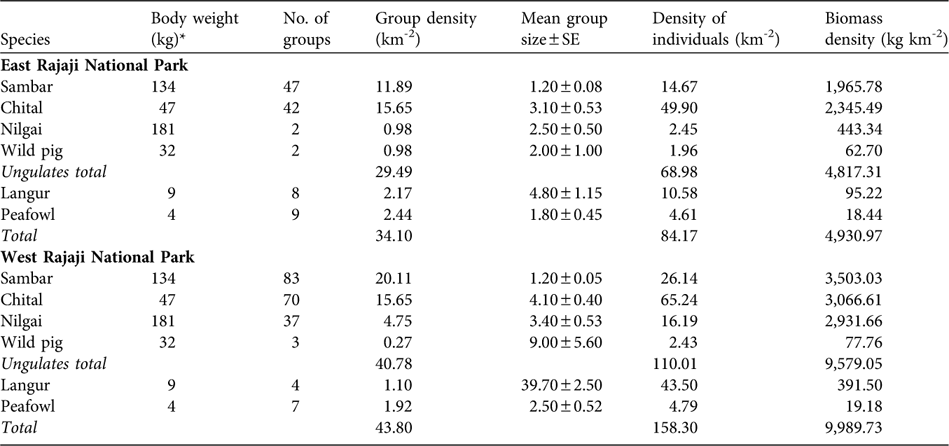
In the eastern sector we estimated densities for six (sambar, chital, nilgai, wild pig, common langur and peafowl) of the 13 prey species that were detected on transects (Table 2), with an estimated average species density of 84.2 km-2 and with c. 81% comprising wild ungulates (69.0 km-2). In terms of group density, chital was the most abundant followed by sambar, peafowl, common langur, nilgai and wild pig. Chital was the most abundant in terms of individual density, followed by sambar, common langur, peafowl, nilgai and wild pig. The estimated ungulate wild prey biomass density was 4,817.31 kg km-2. Of the groups, 13.5% were of small bodied animals (peafowl and common langur, < 20 kg); 48.7% were of medium sized animals (chital and wild pig, 20–50 kg) and 37.8% were of large bodied animals (sambar and nilgai, > 50 kg).
Table 2 Body weight, number of groups, group density, mean group size, density of individuals and biomass density of tiger prey species in the eastern and western sectors of Rajaji National Park (Figs 1–2) during December 2006 to February 2007 (see text for further details).
* From Schaller (Reference Schaller1967) and Karanth & Sunquist (1992)
In the western sector densities were estimated for six (sambar, chital, nilgai, wild pig, common langur and peafowl) of the 11 prey species detected on transects (Table 2), with an average species density of 158.3 individuals km-2 and with wild ungulates (110.01 km-2) contributing 69% of the total density. Group density of sambar was highest followed by chital, nilgai, peafowl, common langur and wild pig. Chital was found to be the most abundant prey species in terms of individual density, followed by common langur, sambar, nilgai, peafowl and wild pig. We estimated the ungulate wild prey biomass density to be 9,579.05 kg km-2. Of the groups, 6.9% were small bodied animals, 36.4% medium sized, and 56.8% large bodied.
Density of tiger
In the eastern sector the total sampling effort amounted to 600 trap nights. Forty camera traps and 15 nights of trapping at each station resulted in a total of 14 photographs of five adult tigers and two cubs (the latter were excluded from the analysis). The closure test supported the assumption that the sampled population was closed for the 60-day study interval (z = -0.390, P = 0.34809). The capture probability (p) was estimated to be 0.1556 and the estimated population size (![]() ± SE) was 6.00 ± 2.51 under the best selected model, Mh. The camera trap polygon (A) formed using periphery camera traps was 52.65 km2. The boundary strip width (Ŵ ± SE) was estimated to be 1.87 ± 0.50 km and the effective sampled area (A(Ŵ) ± SE) was 116.96 ± 5.30 km2. Thus, the estimated tiger density (
± SE) was 6.00 ± 2.51 under the best selected model, Mh. The camera trap polygon (A) formed using periphery camera traps was 52.65 km2. The boundary strip width (Ŵ ± SE) was estimated to be 1.87 ± 0.50 km and the effective sampled area (A(Ŵ) ± SE) was 116.96 ± 5.30 km2. Thus, the estimated tiger density (![]() ± SE) for the eastern sector was 5.12 ± 0.70 tiger per 100 km2.
± SE) for the eastern sector was 5.12 ± 0.70 tiger per 100 km2.
In the western sector the intensive trapping involving 20 camera traps, covering 42.8 km2 and spanning 15 nights of trapping at each station amounted to 300 trap nights and only one photograph of one individual tigress.
Discussion
The two protected areas of Corbett Tiger Reserve (c. 1,280 km2) and Rajaji National Park (c. 820 km2), constituting only c. 30% of the three Tiger Habitat Blocks within the north-western portion of the Terai-Arc Landscape, have been identified as a potential source population of tigers (Johnsingh, Reference Johnsingh2006). Johnsingh et al. (Reference Johnsingh, Ramesh, Qureshi, David, Goyal and Rawat2004) documented high use by tigers of Corbett Tiger Reserve (c. 41%) and adjoining forest divisions (Ramnagar Forest Division; c. 20.7%) based on frequency of indirect tiger signs along 82 km of survey. In addition, Contractor (Reference Contractor2007) estimated a high density of > 16 tigers per 100 km2 in Corbett National Park (c. 520 km2; part of Corbett Tiger Reserve). This highlights the role of this Reserve as a critical source population. Conversely, Johnsingh et al. (Reference Johnsingh, Ramesh, Qureshi, David, Goyal and Rawat2004) documented a relatively low tiger use within Rajaji National Park (c. 12.9%), most of which was attributed to signs recorded in Dholkhand in the western sector and the eastern sector. However, following the minimization of anthropogenic pressures from within the Chilla range of east Rajaji National Park in 2004, Harihar et al. (Reference Harihar, Pandav and Goyal2008) documented evidence of breeding tigers, suggesting the potential of the eastern sector as a source population.
The difference in sign encounter rates of tigers between the western and eastern sectors of Rajaji National Park indicates that the status of the tiger in the western sector is precarious even though most forest ranges there have been free of Gujjar settlements since 2005 (Hardwar, Dholkhand west, Motichur and Kansrau). As the eastern sector is contiguous with Corbett Tiger Reserve, the documented recovery in Chilla range (Harihar et al., Reference Harihar, Pandav and Goyal2008) could be attributed to the colonization by dispersing individuals from Corbett Tiger Reserve facilitated by the Rajaji-Corbett corridor (Lansdowne Forest Division). Western Rajaji National Park and adjoining multiple use forests (Tiger Habitat Block I) depend, however, on the Chilla-Motichur corridor for connectivity to source populations in Tiger Habitat Block II (Johnsingh et al., Reference Johnsingh, Prasad and Goyal1990). This corridor (c. 3 km2) connecting eastern and western Rajaji National Park across the river Ganga is highly disturbed by developmental activities along the river. The expansion of Haridwar and Rishikesh townships and Raiwala village are causing disturbances along the west bank of the river Ganga. The establishment of Khand gaon settlements (I, II and III) for the Tehri dam evacuees, Raiwala Army camp with an ammunition dump, the Hindustan antibiotic factory on the west bank of river Ganga, and the construction of the 14 km long Rishikesh-Chilla power channel on the east bank of river Ganga have all further severed habitat connectivity.
Apart from the lack of connectivity to source populations, western Rajaji National Park is also disturbed because of the proximity of human habitation to the Park boundary. The hierarchical cluster analysis (Fig. 3) further supports earlier findings of the detrimental effects of anthropogenic disturbance on the use of habitat by tigers in the western sector (Johnsingh et al., Reference Johnsingh, Ramesh, Qureshi, David, Goyal and Rawat2004; Kurien, Reference Kurien2005). While firewood collection and lopping of trees cause habitat degradation, the illegal grazing of livestock within the Park poses the threat of retaliatory poisoning of tiger kills by livestock owners. In addition, grass cutters harvesting bhabar grass Eulaliopsis binata access the interior hilly tracts of the Park, disturbing habitats and stealing fresh tiger kills. Such activities render the area unsuitable for successful tiger reproduction.
Since 2005 the forest ranges of Dholkhand west, Dholkhand east and Hardwar of western Rajaji National Park have been free of Gujjar settlements. Following the resettlement programme, a rau walk exercise carried out by the officer trainees of XXVI Post Graduate Diploma course of Wildlife Institute of India in November 2005, i.e. before our study, encountered tiger pug marks in 13 of 15 sampled raus in west Rajaji National Park (Andheri, Binj, Dholkhand, Guleria, Sampowali, Malawali, Bam, Beribada, Gholna, Harnol, Chidak, Rauli and Ranipur, unpubl. data). The apparent decline in use of this area by tigers is a matter of concern. Johnsingh & Negi (Reference Johnsingh and Negi2003) identified poaching as a threat to tigers and their prey in this area but we did not find any evidence of poaching. Most tiger populations have been poached for their bones, an important component in Traditional Chinese Medicine, and the northern Indian regions have been particularly targeted by organized poachers (Wildlife Protection Society of India, 1998).
Prey distribution and density determine habitat selection by tigers (Miquelle et al., Reference Miquelle, Smirnov, Merrill, Myslenkov, Quigley, Hornocker, Schleyer, Seidensticker, Christie and Jackson1999; Karanth & Stith, Reference Karanth, Stith, Seidensticker, Christie and Jackson1999). Although the tiger can prey on a wide variety of species, average prey size is c. 60 kg. This is obtained predominantly from ungulate species, which contribute up to 75% of the prey biomass requirement of tigers (Sunquist et al., Reference Sunquist, Karanth, Sunquist, Seidensticker, Christie and Jackson1999). While the frequency of occurrence of the principal prey species (sambar, chital, nilgai and wild pig) are similar across Rajaji National Park (Table 1), species’ densities are not (Table 2). Tigers respond numerically to ungulate prey densities (Karanth et al., Reference Karanth, Nichols, Kumar, Link and Hines2004), and we therefore predict that the density of prey species in the eastern and western sectors (68.98 and 110.01 km-2, respectively) could support tiger densities of up to 13 and 22 tigers per 100 km2, respectively, with a 10% biomass offtake (compared to the current density of tigers in the eastern sector of 5.12 100 km-2 and the single tigress camera-trapped in the western sector). Camera trapping data from Dholkhand west (S.P. Goyal & A.J.T. Johnsingh, unpubl. data) revealed that a total of eight individual tigers used the area over 1996–2001. However, the lack of connectivity to source populations, anthropogenic disturbances and the fact that poaching may still be occurring, highlight the dismal scenario of tiger populations in Tiger Habitat Block I.
The need to free the Chilla-Motichur corridor of anthropogenic disturbances to aid the recovery of tiger and elephant Elephas maximus populations at the north-western limit of their range has been recognized for some time (Johnsingh et al., Reference Johnsingh, Prasad and Goyal1990). However, the corridor has been progressively degraded. As tigers in eastern Rajaji National Park are increasing in numbers, with signs of breeding, it is important to identify forest patches where the colonization of dispersing individuals can be facilitated. Western Rajaji National Park, with its currently low tiger density and high density of prey, could serve as ideal dispersal grounds provided the recommendations of Johnsingh et al. (Reference Johnsingh, Prasad and Goyal1990, 2004) are implemented.
As recommended by Johnsingh et al. (Reference Johnsingh, Ramesh, Qureshi, David, Goyal and Rawat2004), the relocation of Khand gaon (settlement III) to a pre-designated site in Dehradun Forest Division is currently under progress. The approval for construction of a 1 km flyover for the road and construction of underpasses below the railway line, both passing through the corridor, is still pending in the Supreme Court. For moving the ammunition dump an alternate site has been identified and the forest land has already been denotified. Gujjars residing in Ghauri range of Rajaji National Park and Shampur range of Hardwar Forest Division, which adjoin the Chilla range, need to be resettled to secure c. 300 km2 of undisturbed habitat along the east bank of the river Ganga for the breeding tigers of Chilla. Strict enforcement of protection laws within western Rajaji National Park, to curb firewood extraction, livestock grazing and bhabar grass collection, would secure c. 600 km2 of undisturbed habitat on the west bank of the river Ganga. In addition, the Rajaji-Corbett corridor needs to be as disturbance-free as possible so as to strengthen further the connectivity to Corbett Tiger Reserve. The combination of these various measures would enable the establishment of a breeding population of tigers in Rajaji National Park, thereby ensuring the long-term conservation of the tiger at its north-western limit in the Indian subcontinent.
Acknowledgements
We thank the Director of the Wildlife Institute of India and the Uttarakhand Forest Department for providing permissions and the ongoing Rajaji monitoring project of the Wildlife Institute of India for logistic support. We also thank the Terai-Arc Landscape project of the Wildlife Institute of India for providing access to maps. This study was funded by Save the Tiger Fund (Grant 2005-0013-027) and supported by the Wildlife Institute of India. Imam, Mumtaj, Ameer and Satpal are thanked for their assistance with fieldwork. Mousumi Ghosh is thanked for her assistance during the preparation of the manuscript, and we thank two anonymous reviewers for helpful comments.
Biographical sketches
Abishek Harihar has a particular interest in the population dynamics of mammals. For the past 3 years he has been monitoring large mammalian prey and predator populations in the Rajaji National Park. Deepika L. Prasad is interested in elephants, human-animal conflict, animal behaviour and effective zoo management. Chandan Ri is particularly interested in avian and herpetological research. Bivash Pandav is currently the programme leader for the Tiger and other Asian Big Cats Programme of WWF–International and is interested in threatened species management. Surendra P. Goyal's research interests include the ecology, biology and ecophysiology of ungulates and small carnivores. He is currently involved in developing Wildlife Forensic Science at the Wildlife Institute of India.







