Introduction
South-east Asia is a global biodiversity hotspot with exceptionally high levels of mammalian richness and endemism (Schipper et al., Reference Schipper, Chanson, Chiozza, Cox, Hoffmann and Katariya2008). Rapid rates of deforestation and widespread poaching have caused precipitous declines in mammals across the region (Duckworth et al., Reference Duckworth, Batters, Belant, Bennett, Brunner and Burton2012), driving regional extinctions of several conservation-priority species (Brook et al., Reference Brook, de Groot, Scott, Boag, Long and Ley2012) and threatening others with global extinction (Tilker et al., Reference Tilker, Long, Gray, Robichaud, Van and Nguyen2017). However, even in this biodiversity hotspot, diversity and threat levels are not uniformly distributed. Subregional centres of endemism are of particular concern from a conservation perspective because small global geographical range is a strong predictor of extinction risk (Cardillo et al., Reference Cardillo, Mace, Gittleman, Jones, Bielby and Purvis2008). To design effective mitigation mechanisms for range-restricted species in endemism hotspots it is imperative that conservation scientists obtain basic information on status and distribution, as well as an understanding of the effect of anthropogenic pressures. This information is especially critical for little-known endemic species that occur in areas that are known to be under threat.
The Annamite Range, a mountain chain straddling the border of Viet Nam and Lao People's Democratic Republic (Lao), has one of the highest concentrations of endemic mammal species in continental South-east Asia (Baltzer, Reference Baltzer2001; Tordoff et al., Reference Tordoff, Timmins, Smith and Mai2003). Several of these species are new to science, highlighting how little is known about the biodiversity of this ecoregion (Sterling & Hurley, Reference Sterling and Hurley2005). All Annamite endemic mammals are facing an extinction crisis. Although habitat loss has been a factor in their decline, the primary threat is intensive poaching through the use of wire snares (Gray et al., Reference Gray, Lynam, Seng, Laurance, Long, Scotson and Ripple2017, Reference Gray, Hughes, Laurance, Long, Lynam and O'Kelly2018) to supply the thriving wildlife trade in Indochina (Sodhi et al., Reference Sodhi, Koh, Brook and Ng2004; Harrison et al., Reference Harrison, Sreekar, Brodie, Brook, Luskin and O'Kelly2016). Although snaring is prohibited by law in both Viet Nam and Lao, enforcement is weak and most protected areas are so-called paper parks that offer little or no protection to conservation-priority species (Brook et al., Reference Brook, de Groot, Scott, Boag, Long and Ley2012). As a result of high demand for wildlife products and lax enforcement, industrial-level commercial snaring is pervasive and has caused widespread empty forest syndrome (Redford, Reference Redford1992) across the entire Indochina region.
The Annamite striped rabbit Nesolagus timminsi is amongst the most understudied of the endemic mammals of this region and is currently categorized as Data Deficient on the IUCN Red List (Abramov et al., Reference Abramov, Timmins, Touk, Duckworth and Steinmetz2008). The species was first discovered by scientists in 1996 from specimens found in a local market in central Lao (Surridge et al., Reference Surridge, Timmins, Hewitt and Bell1999; Averianov et al., Reference Averianov, Abramov and Tikhonov2000) and shortly thereafter was confirmed to occur in neighbouring Viet Nam (Dang et al., Reference Dang, Abramov, Tikhonov and Averianov2001). With a dark dorsal stripe, rust-coloured rump, and short tail and ears, it is unlike any other lagomorph in mainland South-east Asia. The little information that biologists have indicates that the species is restricted to wet evergreen forest with little or no dry season and has no clear elevational preference (Abramov et al., Reference Abramov, Timmins, Touk, Duckworth and Steinmetz2008). The species’ known range extends from the northern to central Annamites (Dang et al., Reference Dang, Abramov, Tikhonov and Averianov2001; Abramov et al., Reference Abramov, Timmins, Touk, Duckworth and Steinmetz2008). Like all terrestrial mammals in the Annamites it is threatened by snaring (Dang et al., Reference Dang, Abramov, Tikhonov and Averianov2001; Abramov et al., Reference Abramov, Timmins, Touk, Duckworth and Steinmetz2008; Tilker et al., Reference Tilker, Long, Gray, Robichaud, Van and Nguyen2017).
Standardized surveys are needed to obtain the basic information necessary to make informed conservation management decisions for the Annamite striped rabbit. However, obtaining robust data on the species has proven challenging, both because of the dense tropical rainforest and rugged terrain where it lives and the fact that densities of all mammals are severely depressed across the Annamites. Automatic camera traps provide an effective way to gather data on the species. We conducted systematic camera trapping across five areas in Viet Nam and Lao with the objective of gathering information on the ecology, distribution and status of the Annamite striped rabbit. Here we present data on the species’ activity patterns and sociality, analyse its distribution and the factors influencing its occurrence using an occupancy model framework, and use an N-mixture framework to estimate local abundance. We discuss the implications of our results for the conservation of this little-known endemic species.
Study area
We conducted camera-trap surveys in a large forest block in the central Annamites landscape spanning both Viet Nam and Lao. The forest is divided into five administrative areas. In Viet Nam, surveys were conducted in three protected areas: Bach Ma National Park and the Thua Thien Hue and Quang Nam Saola Nature Reserves. In Lao, surveys were conducted in the eastern section of Xe Sap National Protected Area and an adjacent ungazetted forest block to the south near the village of Ban Palé (Fig. 1). Together these areas cover c. 900 km2. The study area has a tropical monsoon climate and is characterized by rugged terrain, with elevations of 100–2,000 m. The dominant habitat type is closed-canopy wet evergreen forest. Montane forest occurs at the highest elevations (above c. 1,300 m). Forests in this area have an extensive history of past disturbance, including chemical defoliation during the Viet Nam war (Stellman et al., Reference Stellman, Stellman, Christian, Weber and Tomasallo2003), state-enterprise logging during the 1970s and 1980s (Yen et al., Reference Yen, Ziegler, Huettmann and Onyeahialam2005), and ongoing illegal timber extraction. Bach Ma National Park and the Saola Nature Reserves are surrounded by a densely populated human-modified landscape. By contrast, population density in the Lao sites is low and they do not contain extensive human-modified areas. However, the Lao sites are heavily utilized by Vietnamese poachers, and subject to mining and illegal logging operations (Tilker, Reference Tilker2014). A road bisecting the eastern section of Xe Sap National Protected Area and the Palé area facilitates access from neighbouring Viet Nam. Despite this accessibility, the Palé area (c. 150 km2) is one of the least surveyed areas in the Annamites. The lack of past survey effort could be because of its particularly difficult terrain: elevations are 500–2,000 m (mean = 1,109 ± SD 307), with 59% of the area above 1,000 m, and a mean slope of 25.89 ± SD 10.47 °.

Fig. 1. Camera-trap locations across all study sites in the central Annamites landscape of Viet Nam and Lao.
Levels of active protection are generally low but vary in intensity across the landscape. Since 2011, WWF has provided support to the local government counterparts in protected area management in both Viet Nam and Lao. As part of this initiative, snare-removal teams have been active in the Saola Nature Reserves and eastern Xe Sap National Protected Area. In a 5-year timespan the teams removed > 75,000 snares from the Saola Nature Reserves (Gray et al., Reference Gray, Hughes, Laurance, Long, Lynam and O'Kelly2018). There is no evidence of active patrolling efforts in Bach Ma National Park and Palé. Historically, these sites supported a representative suite of Annamite endemic and near-endemic species, including the saola Pseudoryx nghetinhensis, Owston's civet Chrotogale owstoni, the large-antlered muntjac Muntiacus vuquangensis and Edwards's pheasant Lophura edwardsi (Tordoff et al., Reference Tordoff, Timmins, Smith and Mai2003; Sterling & Hurley, Reference Sterling and Hurley2005). However, intensive snaring has wiped out many terrestrial mammal and bird species (Harrison et al., Reference Harrison, Sreekar, Brodie, Brook, Luskin and O'Kelly2016; Tilker et al., Reference Tilker, Long, Gray, Robichaud, Van and Nguyen2017; Gray et al., Reference Gray, Lynam, Seng, Laurance, Long, Scotson and Ripple2017, Reference Gray, Hughes, Laurance, Long, Lynam and O'Kelly2018).
Methods
Data collection and preparation
Systematic camera trapping was conducted during November 2014–December 2016 in two phases: (1) a coarse-grid phase, in which camera-trap stations were spaced c. 2.5 km apart across the study site, and (2) a fine-grid phase, during which intensive camera trapping was conducted over a smaller area using a clustered design (Fig. 1). Camera traps were stationed along animal trails, ridgelines and water sources to maximize mammal detections. All cameras were placed on trees, 20–40 cm above the ground, were operational 24 hours per day, and were left in the field for a minimum of 60 days. At each station two independent white-flash camera traps (Reconyx Hyperfire Professional PC850; Reconyx, Holmen, USA) were set. The coarse-grid phase was designed to provide data that could be analysed in an occupancy framework. A total of 138 coarse-grid stations were set up across the five sites: 53 stations in Bach Ma National Park, 46 in the Saola Nature Reserves, and 39 in the Xe Sap National Protected Area and Palé area. In this phase, the two cameras at each location were set facing in different directions. For analysis, we treat the two cameras as a single station. We conducted the coarse-grid camera trapping over three consecutive time periods (Table 1).
Table 1. Details of camera-trap surveys conducted in a large forest block in the central Annamites landscape of Viet Nam and Lao (Fig. 1), with survey site, phase, date, number of stations, number of trapping days, number of detections of the Annamite striped rabbit Nesolagus timminsi, and number of detections of mammal and large galliform species.
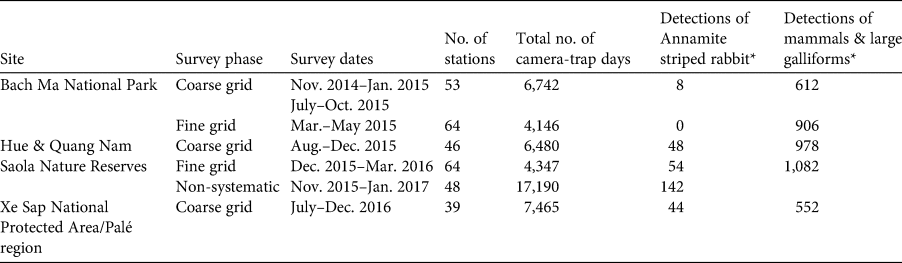
* Δ = 60 minutes.
The fine-grid survey was conducted in core areas of Bach Ma National Park and the Saola Nature Reserves, where a total of 128 individual stations were set up (64 at each location). The stations were arranged in 16 clusters, spaced c. 1.5 km apart. Each cluster consisted of four camera-trap stations, spaced c. 500 m apart and arranged in a square. At each station a pair of cameras was positioned facing each other to photograph both sides of a passing animal. The fine-grid phase was designed to provide data that could be used to estimate density or abundance for species that have individually recognizable markings. Exact fine-grid survey locations within the larger protected areas were chosen to cover areas that appeared to support higher-than-average mammal densities based on information from the coarse-grid dataset.
In addition to the systematic survey, we use data from an opportunistic camera-trap survey (Bushnell Trophy Cam; Overland Park, USA) conducted in the Hue Saola Nature Reserve during November 2015–January 2017, to provide additional information on activity patterns and sociality of the Annamite striped rabbit.
To examine factors that influence Annamite striped rabbit occupancy across our study site we assessed habitat-related and anthropogenic features at both micro- and macro-scales (Table 2). In the field we established a 20 × 20 m grid around the centre point of our coarse-grid stations to demarcate the area for which we collected microhabitat data. We took canopy photographs at the centre point and the corners of the grid to characterize the microhabitat vegetation. To assess vegetation density, we took photographs in each cardinal direction of a 1 × 1.5 m orange sheet positioned 10 m from the centre point. Other in situ habitat features that we measured include the number of bamboo stands, number of dead fallen trees, and number of water sources within the grid. To assess human activity at the camera-trap locations, we recorded human signs. Landscape-level features were evaluated using remote imagery and available geographical information system (GIS) layers. We used 5 m resolution 3A level RapidEye imagery for remote sensing. We used the Random Forest model from the randomForest package in R 3.4.0 (R Development Core Team, 2016) to group imagery into five categories: forest, plantations, degraded areas, bare ground, and water. To evaluate habitat quality we used a weighted mean to calculate forest score, with weights given as follows: plantations, roads and bare areas = 0, degraded areas = 1, and forest = 2. In this classification scheme a higher score corresponds to higher habitat quality. Forest score was calculated within a 50 m radius of each station (following Niedballa et al., Reference Niedballa, Sollmann, bin Mohamed, Bender and Wilting2015).
Table 2. Covariates used for Annamite striped rabbit occupancy modelling.

1Micro-scale covariates were assessed in situ around each camera-trap location; macro-scale covariates were calculated in R using satellite imagery, digital elevation models, and available GIS datasets.
2Anthropogenic covariates are features that may influence hunting pressure directly or indirectly; environmental covariates are features related to habitat quality.
Terrain ruggedness was measured within a 270 m neighbourhood around each station using a shuttle radar topography mission (SRTM) 30 m digital elevation model. We also used the digital elevation model to further enhance water class by creating a hydrology layer using the r.terraflow function in QGIS 2.18.9 (QGIS Development Team, 2016), then calculated Euclidean distance from major streams with package gDistance in R. Euclidean distance to villages and roads was also calculated with gDistance.
Two other anthropogenic factors that could influence Annamite striped rabbit occupancy are current protection levels and past hunting pressure. To evaluate current protection we assigned each study area a binary score: 1 indicates presence of active snare-removal teams and 0 indicates no known patrolling. To approximate past hunting levels we used the number of detections of snaring-sensitive species, defined as all mammals and galliforms with body mass > 500 g, scaled by the number of days the camera trap was active. However, we recognize the possibility that other ecological or sampling-based factors, such as camera-trap placement, may influence detection rates (Sollmann et al., Reference Sollmann, Mohamed, Samejima and Wilting2013). To mitigate this, we derived the detection rate for species not likely to be caught in snares, defined as all mammals and galliforms with body mass < 500 g, and included this as a covariate in our occupancy analyses. If detection rates are driven by camera-trap placement or movement behaviour we would expect a correlation between detections of large and small vertebrates, and between these detection rates and the detection rate of Annamite striped rabbits. However, using a Pearson correlation test we found no strong correlations between any of our detection rates (r < 0.4; Supplementary Fig. 1). Therefore, we conclude that neither camera-trap placement nor similar movement patterns of Annamite striped rabbits and other mammals strongly influenced detectability. As a result we expect that, if detection rates for large vertebrates are reflective of past hunting pressure, only this detection rate would influence Annamite striped rabbit occupancy.
All continuous covariates in the occupancy analyses were standardized to have mean = 0 and SD = 1. We tested for collinearity between all possible pairs of continuous covariates, using Pearson's correlation plots; no covariates were highly correlated (r < 0.7; Supplementary Fig. 2).
Data analysis
All analyses were conducted in R. We used the camtrapR package (Niedballa et al., Reference Niedballa, Sollmann, Courtiol and Wilting2016) to prepare camera-trapping data. To assess Annamite striped rabbit activity patterns we compiled all independent detections (Δ = 60 minutes) and then used the R package overlap to plot a kernel density estimation of daily activity. Sociality was assessed by recording the number of individuals in photographic sequences.
We fitted occupancy models to Annamite striped rabbit detections from the coarse grid. We consider our 60-day trapping period to meet the assumption of closure; spatial independence was confirmed during data analysis (see below). We pooled camera-trap data into 15-day occasions, resulting in at least four sampling occasions for all stations. To determine effort at each station we combined total camera-trap nights for each camera. Detection–non-detection matrices were produced for all stations, with data from the two camera traps at each station combined and analysed as a single station. Single-covariate models were run in the unmarked package (Fiske & Chandler, Reference Fiske and Chandler2011). We first constructed a null model that did not include any covariates and then ran single-covariate models. Because little is known about the species, we were unable to develop a priori hypotheses regarding which factors could influence species occupancy. Therefore, we decided to test each covariate individually to first assess its importance in explaining species occupancy, and only later combine significant covariates into combination models. We included camera-trapping effort as a covariate on detection probability (P). Akaike's information criterion (AIC) was used to rank candidate models (Burnham & Anderson, Reference Burnham and Anderson2003). We considered any model within two ΔAIC units of the top model to be significant. We evaluated the strength of each covariate using P values.
To estimate local abundance of the Annamite striped rabbit at our fine-grid locations we identified individual rabbits based on their unique striping patterns (Supplementary Plate 1). We were thus able to avoid double-counting within a sampling occasion. We pooled camera-trap data into 15-day occasions, then determined the number of individuals detected for each occasion. We used N-mixture models, which estimate site-level abundance using spatially and temporally replicated counts (Royle, Reference Royle2004), to estimate the number of individual rabbits at each camera-trap station. Because no individual rabbit was photographed at two stations, we consider our stations for the fine-grid survey to be spatially independent. As with the occupancy model, we consider our trapping period to approximate temporal population closure. N-mixture models cannot account for heterogeneity in detection among individuals, and therefore resulting abundance estimates may not accurately reflect true abundance (Barker et al., Reference Barker, Schofield, Link and Sauer2018). Nonetheless, the estimates we provide present a meaningful baseline against which to assess future population change (see Discussion). N-mixture analyses were run in unmarked (Fiske & Chandler, Reference Fiske and Chandler2011). Models were run with an underlying Poisson distribution. Although the zero-inflated Poisson and negative binomial may be used when count data are overdispersed, we did not find this to be the case with our dataset.
Results
We obtained data from 138 coarse-grid stations (20,776 camera-trap nights in total) and 128 fine-grid stations (8,404 camera-trap nights in total). We obtained 100 independent detections across 22 stations from the coarse grid, and 54 independent detections across 14 stations from the fine grid. All fine-grid detections occurred in the Saola Nature Reserves, with no photographs from Bach Ma National Park. We identified a total of 27 individuals from the fine-grid data. From 17,190 camera-trap nights in the non-systematic survey in the Hue Saola Nature Reserve we obtained an additional 142 independent detections across 12 stations (Table 1).
The Annamite striped rabbit was primarily solitary. Only two of 296 (< 1%) independent detections showed two individuals in the same photographic sequence. For one of these records, a third individual followed the first two individuals 22 minutes later. All records occurred during 18.00–04.00, with a peak in activity during 01.00–03.00. (Fig. 2), indicating that the species is nocturnal. We recorded the species at elevations of 198–1,304 m (mean = 637 ± SD 290; Fig. 3).

Fig. 2. Kernel density estimate of daily activity patterns for the Annamite striped rabbit Nesolagus timminsi based on camera-trap data. The vertical lines on the x-axis indicate times of individual independent detections (Δ = 60, n = 296), and the grey shading represents night-time.

Fig. 3. Distribution of all camera traps, random sample points (n = 2,000) across the study areas, and detections of the Annamite striped rabbit, by elevation.
Naive occupancy for the Annamite striped rabbit was 0.13 across all sites, and predicted occupancy probability (ψ) for the top-ranked model was 0.18 ± SD 0.04. The probability of detection was 0.36 ± SD 0.05. The top model contained only one covariate, detection rate of mammals and galliforms > 500 g (Table 3; Supplementary Fig. 3). All other models had a ΔAIC > 7 from the top-ranked model. Annamite striped rabbit occupancy was strongly and positively correlated with the number of detections of other hunting-sensitive species (Fig. 4), and all other predictors, including habitat-based covariates, were uninformative in explaining occurrence (Fig. 5).

Fig. 4. Response curve showing change in modelled occupancy (ψ) with detection rates for mammals and galliforms > 500 g.
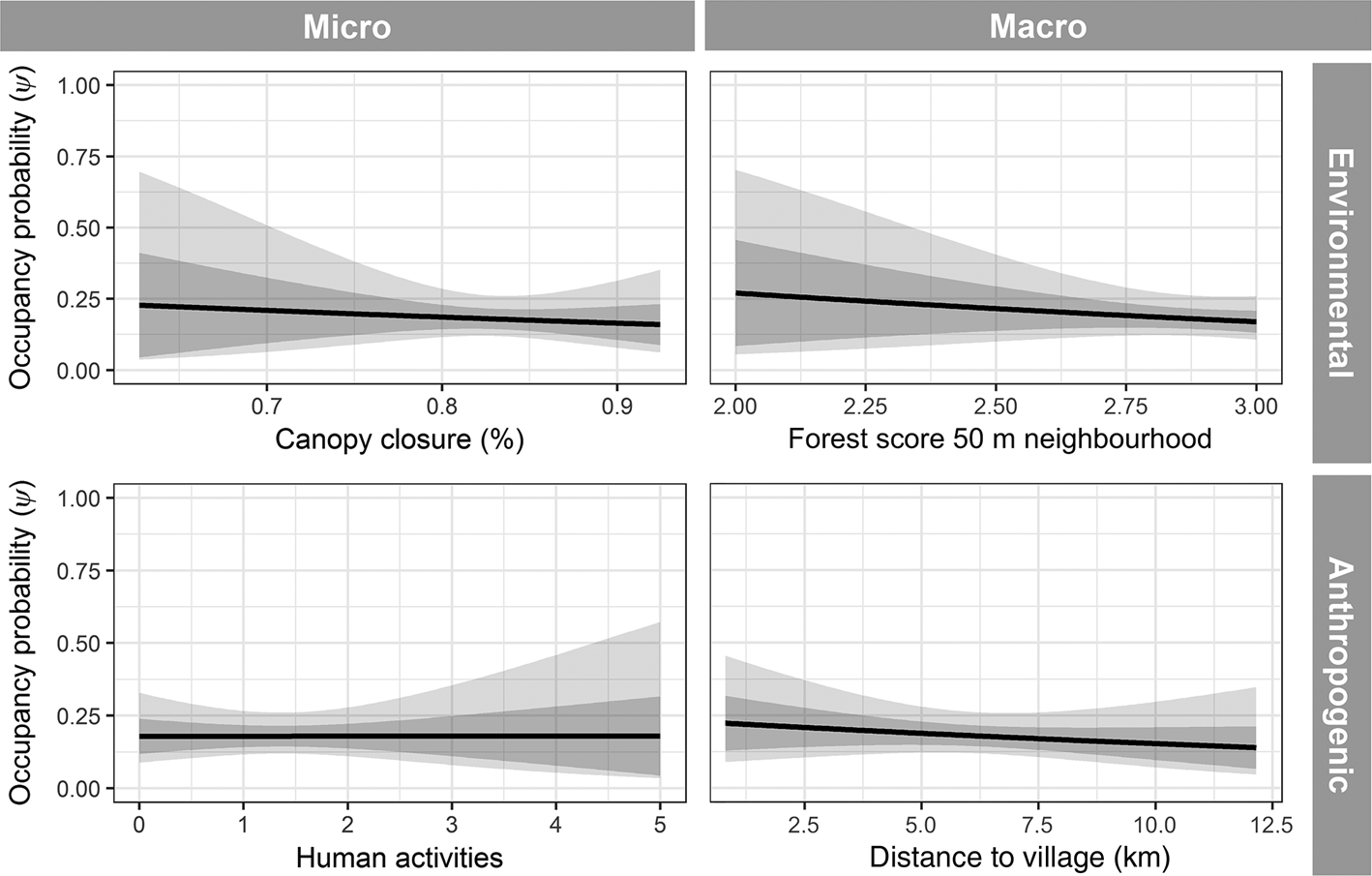
Fig. 5. Annamite striped rabbit occupancy response curves, showing example non-significant results for each representative covariate class.
Table 3. Occupancy model results for the Annamite striped rabbit, ranked by ΔAIC. Bold font indicates significance at P < 0.05.

The N-mixture model estimated a mean local abundance (λ) of 0.57 ± SE 0.20 individuals per station in the Saola Nature Reserves. Detection probability (P) was estimated to be 0.19 ± SE 0.07. With no detections in the Bach Ma National Park fine-grid site, we could not estimate local abundance with N-mixture models. As survey effort in this site was high, we interpret this result as indicating that true local abundance at this site is zero or close to zero.
Discussion
As with many other lagomorphs, the Annamite striped rabbit is nocturnal. Nocturnal activity is often explained by predator avoidance, as many natural predators hunt primarily by sight. The species’ solitary behaviour is also consistent with most leporids (Cowan & Bell, Reference Cowan and Bell1986). The two observed Annamite striped rabbit pairs may indicate behaviour related to breeding. In both sequences, the first rabbit moves through the frame, followed immediately by a second rabbit on a direct path to the first. All individuals were approximately the same size and are unlikely to be parent and offspring. However, because we have so few records of paired individuals and were unable to determine sex from the photographs, it is not possible to draw conclusions related to reproduction behaviour from our data.
Our analysis indicates that the species shows no strong elevational preferences (Fig. 3), which is consistent with the limited information available. Dang et al. (Reference Dang, Abramov, Tikhonov and Averianov2001) reported an individual found in a snare at 200 m elevation but also noted that hunters report the species at higher elevations. Although Abramov et al. (Reference Abramov, Timmins, Touk, Duckworth and Steinmetz2008) suggested that the species can probably utilize areas as low as sea level, we did not record the species at extremely low elevations, possibly because lowland areas are readily accessible and have higher levels of past hunting pressure. The fact that we did not record the species above 1,304 m could indicate that the species does not occur in forest with montane characteristics, although our survey effort at high elevations was limited and therefore additional work is needed to investigate this.
Numerous studies have demonstrated that species occurrence is driven by multiple factors operating at various spatial scales (Gerber et al., Reference Gerber, Karpanty and Randrianantenaina2012; Koerner et al., Reference Koerner, Poulsen, Blanchard, Okouyi and Clark2017). We hypothesized that occurrence of the Annamite striped rabbit would be influenced by a combination of current habitat and anthropogenic factors. Our results show that detections of other snaring-sensitive species, which we interpret as an indication of past hunting pressure, is the best predictor of occurrence of the Annamite striped rabbit in our study sites. The failure of other predictors to explain occupancy was unexpected. We believe that this finding is explained by the fact that past hunting pressure has overwhelmed environmental relationships. In this scenario, the Annamite striped rabbit would, in the absence of excessive hunting pressure, be more widely distributed and abundant in the landscape. As a result of the overwhelming snaring pressure in the Annamites (Gray et al., Reference Gray, Lynam, Seng, Laurance, Long, Scotson and Ripple2017, Reference Gray, Hughes, Laurance, Long, Lynam and O'Kelly2018), several endemic mammal species, including the saola, have distributions determined almost exclusively by hunting pressure (Timmins et al., Reference Timmins, Hedges and Robichaud2016b). We postulate that the Annamite striped rabbit follows a similar distribution pattern within our study site. Given the range of environmental covariates we measured, and the fact that occupancy is strongly correlated to presence of other hunting-sensitive species, we suggest that the Annamite striped rabbit exhibits characteristics of a refugee species whose distribution is driven by anthropogenic pressures rather than habitat preferences (Kerley et al., Reference Kerley, Kowalczyk and Cromsigt2012). Further studies are needed to investigate whether the Annamite striped rabbit also utilizes suboptimal but safer habitats, a pattern that has been observed for other refugee species (Bocherens et al., Reference Bocherens, Hofman-Kamińska, Drucker, Schmölcke and Kowalczyk2015).
Local abundance patterns from our fine-grid data indicate that the Annamite striped rabbit is extirpated locally in the central part of Bach Ma National Park, and present at low to moderate densities in the Saola Nature Reserves. This finding is generally consistent with our coarse-grid data, which indicated the species was present but rare across the National Park, but not uncommon in the Saola Nature Reserves. Although intensive defaunation has occurred across all parts of this landscape, Bach Ma National Park appears to be emptier than the neighbouring Saola Nature Reserves, based on detections of other hunting-sensitive species (Table 1). In addition, the Saola Nature Reserves have some level of active anti-poaching protection, whereas enforcement efforts in Bach Ma are extremely low. We suggest that more intensive snaring in Bach Ma, exacerbated by the lack of enforcement efforts, has led to the probable extirpation of the species from large parts of the protected area. The difference in local abundances between two areas with similar habitat offers additional evidence for occurrence patterns driven by hunting.
As this is the first study to estimate local abundance (λ) for the species, we cannot compare our estimates for the Saola Nature Reserves to past studies. However, because the Saola Nature Reserves have experienced heavy hunting pressure, we believe that the local abundance is well below what would exist under undisturbed conditions. Although no area in the Annamites landscape is unaffected by snaring, there are sites within the species’ range that, on the basis of expert assessments and limited camera trapping, have not been as heavily affected as our study sites. We recommend additional camera trapping at these sites, to obtain comparable local abundance estimates for the Annamite striped rabbit. It is likely that only surveys in less hunted areas, where the impact of hunting on distribution is weaker, will be able to offer insight into the ecology of the species.
Our results provide information that is directly applicable to Annamite striped rabbit conservation. The camera trapping provides spatially explicit data that can be used for targeted anti-poaching work. With the complete or functional extinction of numerous flagship mammal species from the Annamites, including regionally important species such as the Javan rhinoceros Rhinocerous sondaicus (Brook et al., Reference Brook, de Groot, Scott, Boag, Long and Ley2012) and tiger Panthera tigris (Walston et al., Reference Walston, Robinson, Bennett, Breitenmoser, da Fonseca and Goodrich2010), and endemics such as the saola (Tilker et al., Reference Tilker, Long, Gray, Robichaud, Van and Nguyen2017), the Annamite striped rabbit may be the highest priority terrestrial mammal species with sizeable populations remaining at our study sites. We therefore recommend that current anti-poaching patrols concentrate snare-removal efforts in locations where the species was recorded. Our data further support an ongoing initiative by conservation stakeholders to gazette the Palé area as a protected area. The occurrence of the Annamite striped rabbit in multiple locations within this area, along with other conservation-priority species such as Owston's civet and the Asiatic black bear Ursus thibetanus (Tilker, Nguyen & Wilting, unpubl. data), underscores the importance of this area for conservation.
Ultimately, conservation stakeholders need to embed species conservation programmes into a holistic, adaptive management framework (Keith et al., Reference Keith, Martin, McDonald-Madden and Walters2011). Assessing population trends over time is a key component of adaptive management and requires a baseline for assessment of future changes. Here we establish two baselines for the Annamite striped rabbit: at the landscape scale and at a local scale within the Saola Nature Reserves. For long-term adaptive management of the species in this landscape we recommend repeating the systematic camera trapping in the near future to assess possible changes in the population within these areas in response to continued snare-removal efforts.
Several Annamite endemic species are facing imminent extinction as a result of intensive snaring. The saola is so rare that conservation breeding is now believed to be the last hope to save the species (Tilker et al., Reference Tilker, Long, Gray, Robichaud, Van and Nguyen2017). The large-antlered muntjac and Edwards's pheasant are both Critically Endangered (BirdLife International, 2016; Timmins et al., Reference Timmins, Duckworth, Robichaud, Long, Gray and Tilker2016a), and the latter may already be extinct in the wild (Grainger et al., Reference Grainger, Ngoprasert, McGowan and Savini2017). The status of the Annamite striped rabbit is not as severe but the lack of records from Bach Ma National Park is cause for concern. The status and population trend of the Annamite striped rabbit in this protected area, although better than for other endemic species that have probably become extirpated there in recent years (including the saola and large-antlered muntjac), appears to be following the trajectory of these hunting-sensitive species (R. Timmins, pers. comm., 2018). Without immediate and effective anti-poaching efforts, the Annamite striped rabbit may become as rare as the saola.
Acknowledgements
We thank the staff of the WWF-CarBi project for providing extensive logistical support; Bach Ma National Park, the Thua Thien Hue and Quang Nam Saola Nature Reserves, and Xe Sap National Protected Area for providing permissions and personnel to conduct this research; and our field team leaders in Viet Nam and Lao. Funding for the surveys was provided by the German Federal Ministry of Education and Research (BMBF FKZ: 01LN1301A), Leibniz-IZW, Point Defiance Zoo and Aquarium, Safari Club International, and Critical Ecosystem Partnership Fund. AT received support through a Fulbright scholarship. The CarBi project was provided by Internationale Klimaschutzinitiative of the Federal Ministry for the Environment, Nature Conservation, and Nuclear Safety (BMBU) and Kreditanstalt für Wiederaufbau (KfW). AT would like to thank Rob Timmins for discussions about the Annamite striped rabbit, and Barney Long, Tom Gray, and Wes Sechrest for support while working in the Annamites. We thank two anonymous reviewers whose input substantially improved this article.
Author contributions
Design of systematic camera-trap survey: AT, RS, AW; fieldwork for systematic camera-trap survey: AT, AN; design of non-systematic survey: ML; fieldwork for non-systematic survey: TVN, ATN; analysis of survey data: AT, JFA; analysis of remote-sensing data: TB, JN; writing: AT, JFA, AW; revision of article: all authors.
Conflicts of interest
None.
Ethical standards
This research complies with the Oryx Code of Conduct.










