Introduction
The jaguar Panthera onca is the largest felid of the Americas. During the 20th century its distribution was reduced to almost half of its original range (Sanderson et al., Reference Sanderson, Redford, Chetkiewicz, Medellin, Rabinowitz, Robinson and Taber2002). This reduction was particularly severe in Argentina (Perovic, Reference Perovic2002) and the species now occurs in only three isolated areas in the north (Altrichter et al., Reference Altrichter, Boaglio and Perovic2006; Di Bitetti et al., Reference Di Bitetti, De Angelo, Paviolo, Schiaffino, Perovic, Corchera and Brown2006a). The jaguar is considered a threatened species in Argentina (Díaz & Ojeda, Reference Díaz and Ojeda2000), and is categorized as a National Natural Monument (Ley No. 25463, 2001). In Brazil it is considered threatened (Reis et al., Reference Reis, Peracchi, Pedro and Lima2006) and it has disappeared from most of the Atlantic Forest, where only a few small and isolated populations remain (Leite et al., Reference Leite, Boulhosa, Galvao, Cullen, Medellín, Equihua, Chetkiewicz, Crawshaw, Rabinowitz and Redford2002; Cullen et al., Reference Cullen, Abreu, Sana and Ferreira Dales Nava2005).
Globally the Atlantic Forest of South America is one of the most threatened rainforests. Distributed across north-east Argentina, south-west Brazil and eastern Paraguay, the Upper Paraná Atlantic Forest is the innermost region of the Atlantic Forest complex (Di Bitetti et al., Reference Di Bitetti, Placci and Dietz2003) but is being reduced and fragmented as a result of its conversion to other land uses (Holz & Placci, Reference Holz, Placci, Galindo-Leal and Câmara2003).
The largest remnant of the Upper Paraná Atlantic Forest, known as the Green Corridor (c. 10,000 km2, Fig. 1a) lies in the Misiones Province of Argentina and neighbouring areas of Brazil. The Corridor holds the southernmost population of jaguars and has been identified as a high priority area for the conservation of the species because of its long-term conservation potential and its ecological uniqueness (Sanderson et al., Reference Sanderson, Redford, Chetkiewicz, Medellin, Rabinowitz, Robinson and Taber2002; Marieb, Reference Marieb2006). This jaguar population is the only one that has received that category in Argentina and in the Atlantic Forests (Eizirik et al., Reference Eizirik, Indrusiak, Johnson, Medellín, Equihua, Chetkiewicz, Crawshaw, Rabinowitz and Redford2002; Sanderson et al., Reference Sanderson, Redford, Chetkiewicz, Medellin, Rabinowitz, Robinson and Taber2002)
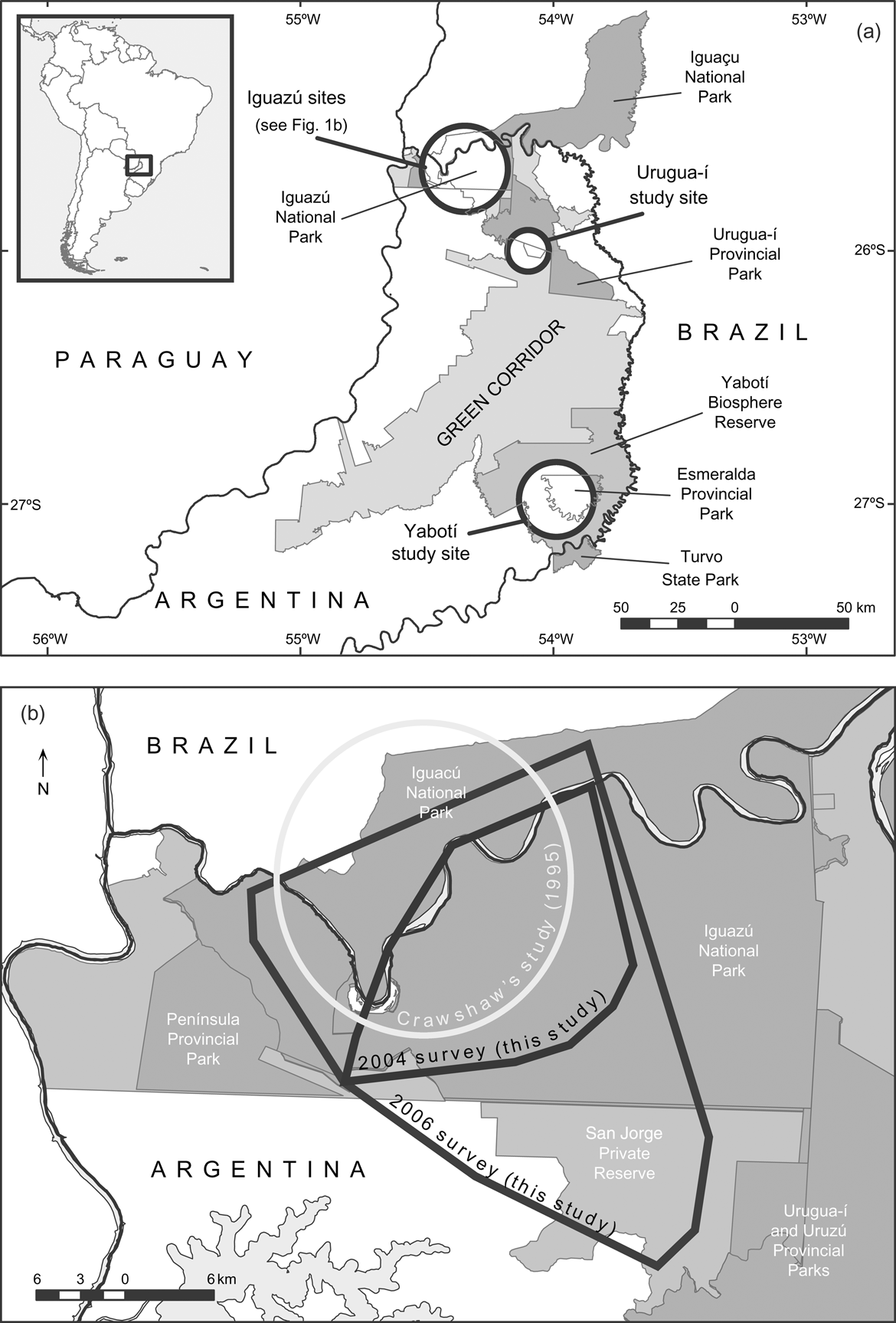
Fig. 1 (a) The Green Corridor of Argentina and Brazil and its main protected areas; the circles indicate the three study sites. The rectangle on the inset illustrates the location of the main map at the border between Argentina and Brazil. (b) The Iguazú study site, showing the minimum convex polygons that include all the camera-trap stations during the surveys of 2004 and 2006, and Crawshaw's (Reference Crawshaw1995) study area.
.
Crawshaw (Reference Crawshaw1995) provided relevant ecological information for this jaguar population, including home range size, population density and diet, and identified important threats. However, the decline in the rate of observed jaguar signs and in the number of reported attacks on domestic animals suggest that its population could have declined since the 1990s (Crawshaw, Reference Crawshaw, Medellín, Equihua, Chetkiewicz, Crawshaw, Rabinowitz and Redford2002).
The goal of this study was to estimate jaguar population densities widely across the Green Corridor and thus make a robust estimate of population size, assess the species' conservation status, and compare the population estimate with earlier values from the region (Crawshaw, Reference Crawshaw1995; Cullen et al., Reference Cullen, Abreu, Sana and Ferreira Dales Nava2005) to assess population trends.
Study area
The Green Corridor is dominated by semi-deciduous forest and has a humid subtropical climate with hot summers (December–March) and winters with frosts (June–August). Mean total annual precipitation is 1,700–2,200 mm, with no marked dry season (Crespo, Reference Crespo1982).
We conducted the first camera-trap survey at Urugua-í in 2003 in an area that comprises the Urugua-í Private Wildlife Reserve (32 km2), a portion of the Urugua-í Provincial Park (840 km2) and a portion of Campo Los Palmitos (300 km2), which belongs to a timber company and contains old pine (Pinus taeda and Pinus ellioti) plantations in a matrix of native forest (Fig. 1a). This forest was selectively logged until the beginning of the 1990s but is in a relatively good condition (Di Bitetti et al., Reference Di Bitetti, Paviolo and De Angelo2006b). Lack of resources precludes effective control of poaching, and the area suffers a moderate to high hunting pressure (Table 1).
Table 1 Area, law enforcement capacity and evidence of poaching in the three surveyed areas (Iguazú was surveyed in both 2004 and 2006; Fig. 1).
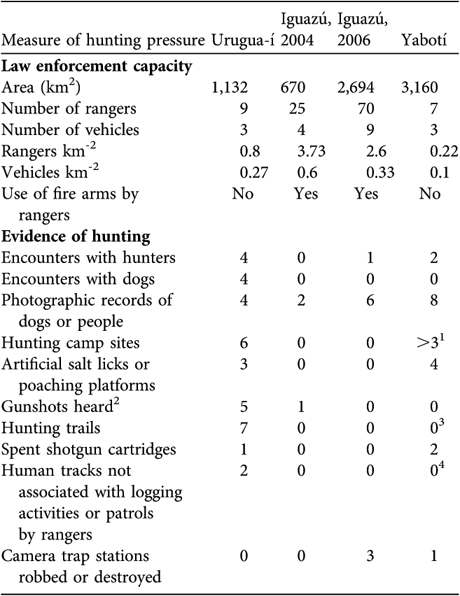
1 This is an underestimate. We found three hunters' camp sites but there were many logging camps, employees of which usually hunt at the weekends. On the two occasions we found spent shotgun cartridges it was at logging camps.
2 Number of independent events, at each of which we may have heard > 1 shot.
3 At Yabotí we opened few trails and this reduced the likelihood of crossing or detecting hunting trails.
4 Most private properties were under logging operations and we were not able to distinguish tracks of workers from those of poachers.
The second study site is Iguazú, which we surveyed twice, in 2004 and in 2006–2007. The first survey comprised the central area of the Iguazú National Park of Argentina (670 km2, Fig. 1). This is the best-protected area of the region (Giraudo et al., Reference Giraudo, Krauczuk, Arzamendia, Povedano, Galindo-Leal and Câmara2003) and suffers a relatively low hunting frequency, restricted mainly to the park edges (Table 1). In the second survey we expanded the sampled area to cover most of Iguazú National Park, the whole San Jorge Forest Reserve and the western area of the Brazilian Iguaçu National Park of 1,850 km2 (Fig 1b). The surveyed area overlaps extensively with Crawshaw's (Reference Crawshaw1995) study area (Fig. 1b). The absence of a buffer zone and the extensive edge of the western portion of the Brazilian Park makes this area accessible to poachers (Crawshaw, Reference Crawshaw, Medellín, Equihua, Chetkiewicz, Crawshaw, Rabinowitz and Redford2002). The San Jorge Forest Reserve (174 km2) is owned by a timber company and is covered by native forest that was selectively logged until 20 years ago (O. Lescano, pers. comm.).
The third study site is in the Yabotí Biosphere Reserve (2,600 km2), which we surveyed in 2005, and comprises private properties with forest that are being currently logged (2,200 km2). It also comprises Esmeralda Provincial Park (300 km2, logged until 1990). Poachers frequently arrive from villages located outside the reserve and from the Brazilian border. Anti-poaching control is insufficient and the area suffers moderate to high hunting pressure (Table 1).
Methods
To estimate jaguar densities we used a standard camera-trapping protocol (Karanth, Reference Karanth1995; Karanth & Nichols, Reference Karanth and Nichols1998) that has been used to estimate jaguar densities (Wallace et al., Reference Wallace, Gomez, Ayala and Espinoza2003; Maffei et al., Reference Maffei, Cuéllar and Noss2004; Silver et al., Reference Silver, Ostro, Marsh, Maffei, Noss and Kelly2004; Cullen et al., Reference Cullen, Abreu, Sana and Ferreira Dales Nava2005; Soisalo & Cavalcanti, Reference Soisalo and Cavalcanti2006; Salom-Pérez et al., Reference Salom-Pérez, Carrillo, Sáenz and Mora2007). The method makes use of capture-mark-recapture closed population models to estimate animal densities (Otis et al., Reference Otis, Burnham, White and Anderson1978). Individuals are identified in photographs by their distinctive spotted coat. From the capture-recapture history of individuals we estimated population size (Karanth, Reference Karanth1995).
At each study site we set 34–47 sampling stations (Table 2), each of which consisted of a pair of camera traps operating independently and facing each other. The stations were located at regular intervals on trails and rarely-used dirt roads. Prior to the full survey period we conducted preliminary surveys of 4–7 months to identify promising sites for placing the stations. Each full survey lasted 96 days. During this period we set up camera traps in half of the sampling stations for the first half of the survey period (day 1–(45–50)), after which we moved the stations to the remaining sampling stations for the rest of the study period (day (45–50)–96).
Table 2 Number of camera-trap stations, mean distance between stations, date of the full survey, and survey effort for full and for both surveys combined, in the three surveyed areas (Iguazú was surveyed in both 2004 and 2006; Fig. 1).
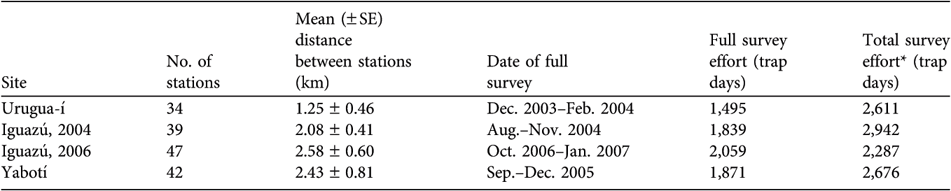
* Includes effort of the preliminary and full surveys
In Urugua-í and Yabotí we could not estimate the abundance of jaguars by means of capture-recapture population models because of the small number of individuals recorded. In these sites we provide an estimate of a minimum number of individuals present in the area from the photographs obtained by camera traps and the observation of jaguar tracks. To estimate jaguar abundance at Iguazú we used the software CAPTURE (Rexstad & Burnham, Reference Rexstad and Burnham1991), which provides population estimates using various models (Otis et al., Reference Otis, Burnham, White and Anderson1978; White et al., Reference White, Anderson, Burnham and Otis1982). We report the results of model Mh using a Jackknife estimator. Mh assumes heterogeneity among individuals in their capture probabilities and is the most appropriate model because of the unequal access to sampling stations by different individuals (Karanth & Nichols, Reference Karanth and Nichols2002). We divided the survey period into 16 trapping occasions of 6 consecutive days each with the aim of having a capture probability higher than 0.1, as recommended by Otis et al. (Reference Otis, Burnham, White and Anderson1978) and White et al. (Reference White, Anderson, Burnham and Otis1982). Finally, we performed the closure test provided by CAPTURE to test the closed population assumption.
To estimate density it is necessary to calculate the area effectively sampled. This is usually accomplished by applying to each sampling station a buffer equivalent to ½ of the mean maximum distance of recaptures (MMDM) for the individuals recorded at more than one station (Silver et al., Reference Silver, Ostro, Marsh, Maffei, Noss and Kelly2004). However, in some situations using ½ MMDM as the buffer could inflate density estimates (Trolle & Kéry, Reference Trolle and Kéry2003; Soisalo & Cavalcanti, Reference Soisalo and Cavalcanti2006; Maffei & Noss, Reference Maffei and Noss2007) and, if radio-telemetry data exist, the best buffer should be the radius of the estimate of mean home range (Soisalo & Cavalcanti, Reference Soisalo and Cavalcanti2006). We provide three density estimates using different buffers to estimate the effectively sampled areas: (1) the radius of the mean adult home range (n = 3 individuals) from Crawshaw (Reference Crawshaw1995); (2) ½ MMDM; (3) MMDM. Because of the small number of jaguars photographed we calculated MMDM as the mean maximum distance moved by all the individuals with recaptures at more than one station, pooling the data from the four surveys (n = 8). For individuals that were captured (and recaptured at > 1 station) in the two surveys at Iguazú (n = 2), we averaged their maximum distance moved (MDM) for both surveys, thus contributing only one value each to the estimate of MMDM. After applying the buffer to the sampling stations we subtracted, from the total area thus obtained, the portions of unsuitable jaguar habitat (e.g. cities, annual crops, airports) to estimate the effectively surveyed area. We used the geographical information system ArcView v. 3.2 (ESRI, Redlands, USA) to estimate MMDM values and surveyed areas.
We used an ANOVA, and post hoc comparisons among samples using the Tukey-Kramer test, to compare our estimates of jaguar density with others for the same region (Iguazú: Crawshaw, Reference Crawshaw1995; Morro do Diabo State Park: Cullen et al., Reference Cullen, Abreu, Sana and Ferreira Dales Nava2005) and with other published estimates from across the jaguar's range (Ceballos et al., Reference Ceballos, Chávez, Rivera, Manterota, Wall, Medellín, Equihua, Chetkiewicz, Crawshaw, Rabinowitz and Redford2002; Nuñez et al., Reference Nuñez, Miller, Lindzey, Medellín, Equihua, Chetkiewicz, Crawshaw, Rabinowitz and Redford2002; Maffei et al., Reference Maffei, Cuéllar and Noss2004; Silver et al., Reference Silver, Ostro, Marsh, Maffei, Noss and Kelly2004; Soisalo & Cavalcanti, Reference Soisalo and Cavalcanti2006; Salom-Pérez et al., Reference Salom-Pérez, Carrillo, Sáenz and Mora2007). For these comparisons we used our estimates obtained using ½ MMDM as the buffer. For Iguazú we used only one density value (the mean of the two surveys). After checking for deviations from normality we performed the ANOVA with ln(density).
To estimate the population of adult jaguars in the Green Corridor we first appraised the area of potential jaguar habitat by using detailed jaguar presence information obtained in 4 years of intensive large-scale census of jaguar presence (C.D. De Angelo, unpubl. data), and then digitized forest and plantation cover that surrounded jaguar presence points from 2004 Landsat satellite images using ArcView. We categorized this potential area into three habitat categories: (1) not suitable (jaguars absent), (2) well protected, and (3) poorly protected. Finally, we extrapolated our maximum and minimum density estimates for areas with low protection (mean of Yabotí and Urugua-í) and high protection (mean of the Iguazú surveys) to the available area in categories 2 and 3 of habitat quality to estimate the number of jaguars.
Results
We photographed 13 different adult jaguars during the four surveys, eight at more than one station. The maximum (25.08 km) and minimum (2.35 km) distances of recapture were for an adult male and female, respectively, at Iguazú. The MMDM was 11.33 ± SE 2.7 km (n = 8), which is not statistically different from the mean diameter of the home ranges of the adult jaguars estimated by Crawshaw (Reference Crawshaw1995; 8.55 ± SE 1.45 km, n = 3; ANOVA F = 0.36, P = 0.563).
Urugua-í We recorded only one individual, an adult male, captured twice (at two sampling stations; MDM = 9.44 km) during the full survey and once during the preliminary survey. Jaguar tracks were found only three times and probably belonged to the photographed jaguar, because they were similar in size. Considering the low occurrence of tracks and the low number of photographic captures (Table 3), it is possible that the animal photographed could have been the only resident jaguar in the surveyed area. The effectively sampled area varied widely (299.01–823.63 km2) depending on the buffer used. We estimate a minimum density of 0.12–0.33 per 100 km2 at Urugua-í (Table 2).
Table 3 Number of jaguar photo-captures per 1,000 trap-days, number of adult jaguars recorded, population estimate, buffer applied for calculations (see text for further details), area surveyed, and density estimates for each of the four full camera-trap surveys.
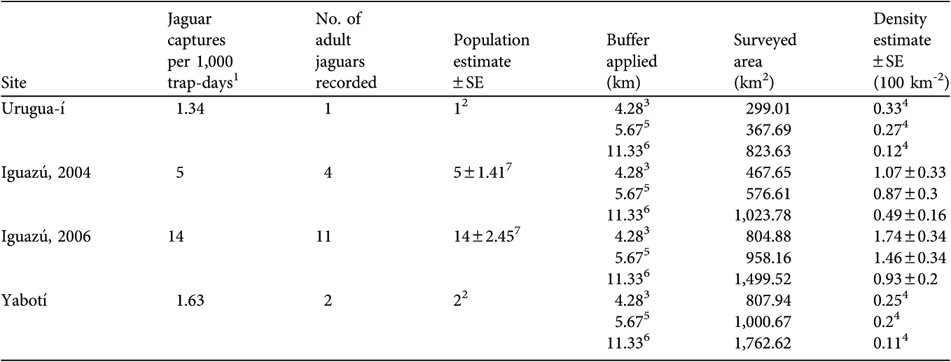
1 > 1 h had to pass for consecutive photographs of a jaguar to be considered independent records
2 Number of jaguars recorded in the study area during the survey
3 ½ of the radius of the mean adult home range estimates (n = 3) from Crawshaw (Reference Crawshaw1995)
4 Minimum density estimate
5 ½ of the mean maximum distance of recapture (½ MMDM) for all the individuals recaptured at > 1 sampling station during the four surveys
6 Mean maximum distance of recapture (MMDM) for all individuals recaptured at > 1 sampling station during the four surveys
7 Abundance estimate obtained with CAPTURE (Rexstad & Burnham, Reference Rexstad and Burnham1991) using model Mh
Iguazú During the first full survey we obtained 10 photographs of four different adults (three females, one male). We also photographed two other jaguars that were not included in the analyses because they were captured outside the full survey period of 96 days. CAPTURE indicate that this population is not different from a closed one (z = -0.942, P = 0.173) and estimated a population of 5 ± SE 1.41 adults. The effectively sampled area was 467.65–1,023.78 km2, depending on the buffer used (Table 3). We estimated an adult jaguar density of between 0.49 ± SE 0.16 and 1.07 ± SE 0.33 per 100 km2 (Table 3). During the second survey we obtained 28 photographs of 11 different adult individuals (six females, four males, one unsexed individual), four of which were photographed during the 2004 survey. Two of the females were photographed with large cubs (c. 1 year old). CAPTURE indicated there was no violation of the closed population assumption (z = -1.392, P = 0.082) and estimated an adult population of 14 ± SE 2.45. The estimated sampled area was 804.88–1,499.52 km2, depending on the buffer. We estimated an adult jaguar density of between 0.93 ± SE 0.2 and 1.74 ± SE 0.34 per 100 km2 (Table 3).
Yabotí We photographed only one male, recorded once and three times during the preliminary and full surveys, respectively. We recorded large tracks (right front track 12.1 cm long × 13.1 cm wide), most probably belonging to the photographed male, and medium size tracks (right front track 9.1 cm long × 10.6 cm wide) probably from an adult female or a subadult male. Thus, the surveyed area was occupied by at least two jaguars, giving a minimum estimate of 0.11–0.25 per 100 km2 (Table 2).
There are statistically significant differences between the estimated jaguar densities at the three study sites and those obtained in the same area > 10 years before or in other portions of the same region (n = 2) and with other jaguar studies (n = 10; ANOVA F 2,12 = 17.49, P = 0.0003; Fig. 2). Tukey-Kramer comparisons among samples indicated that the jaguar densities of the Green Corridor (mean = 0.545 ± SE 0.31 per 100 km2) are lower than those of other sites outside the Upper Paraná Atlantic Forest (5.243 ± SE 0.77 per 100 km2) and those previously obtained in the Green Corridor or in other fragments of the Upper Paraná Atlantic Forest (2.96 ± SE 0.74 per 100 km2). The latter two estimates are not statistically different.
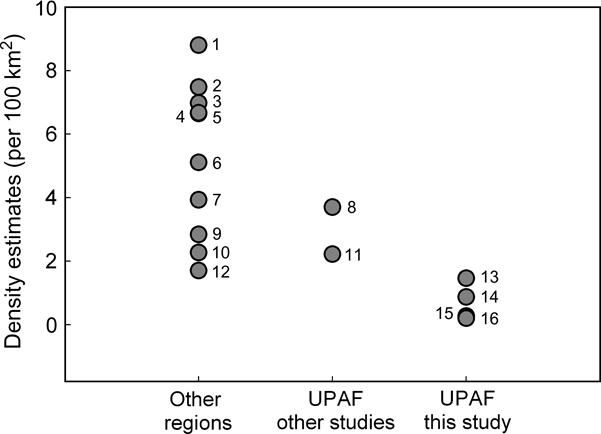
Fig. 2 Estimates of jaguar density in the Upper Paraná Atlantic Forest (UPAF) from this (13, 14, 15, 16) and other studies (8, Iguazú: Crawshaw, Reference Crawshaw1995; 11, Morro do Diabo State Park: Cullen et al., Reference Cullen, Abreu, Sana and Ferreira Dales Nava2005), and from other regions (1, 2, 6, 7, 9, Silver et al., Reference Silver, Ostro, Marsh, Maffei, Noss and Kelly2004; 3, Salom-Pérez et al., Reference Salom-Pérez, Carrillo, Sáenz and Mora2007; 4, Ceballos et al., Reference Ceballos, Chávez, Rivera, Manterota, Wall, Medellín, Equihua, Chetkiewicz, Crawshaw, Rabinowitz and Redford2002; 5, Soisalo & Cavalcanti, Reference Soisalo and Cavalcanti2006; 10, Maffei et al., Reference Maffei, Cuéllar and Noss2004; 12, Nuñez et al., Reference Nuñez, Miller, Lindzey, Medellín, Equihua, Chetkiewicz, Crawshaw, Rabinowitz and Redford2002).
We calculated an area of 9,234 km2 of habitat with potential jaguar presence in the Green Corridor. The extrapolation of the minimum and maximum jaguar density estimates to the available areas of high and low protection results in an estimate of 25–53 adult jaguars in the Green Corridor.
Discussion
Jaguars currently occur at low densities in the Green Corridor of Argentina and Brazil. Our estimates of density are the lowest obtained for the species across its range, and lower than those obtained by Crawshaw (Reference Crawshaw1995) in the same area and by Cullen et al. (Reference Cullen, Abreu, Sana and Ferreira Dales Nava2005) in the northern part of the same region. We believe we have documented a decline that has taken place since the early 1990s. At Iguazú our estimates are 2–7.5 times lower than the 3.7 jaguars per 100 km2 calculated by Crawshaw in 1995 (Fig. 2). The diminution of jaguar signs (Crawshaw, Reference Crawshaw, Medellín, Equihua, Chetkiewicz, Crawshaw, Rabinowitz and Redford2002) and fewer recorded attacks by jaguar on domestic animals during the last few years (K. Schiaffino, pers. comm.) also suggest that jaguars are now less abundant.
Our low density estimates in comparison to other studies could have resulted from methodological differences or sampling artefacts but ancillary evidence suggests our estimates are reliable. Firstly, our sampling effort was similar or greater than that of other studies in terms of the number of stations and trap-days, and our camera-trap surveys at Yabotí and Iguazú are probably the largest ever conducted for jaguars in terms of the area sampled (A. Noss, pers. comm.). Secondly, although Crawshaw (Reference Crawshaw1995) used radio-telemetry other studies estimated similar jaguar densities using both radio-telemetry and camera trapping in the same region (Cullen et al., Reference Cullen, Abreu, Sana and Ferreira Dales Nava2005), and our ocelot density estimates for Iguazú using camera traps are not different from Crawshaw's (Reference Crawshaw1995) estimate using radio-telemetry (Di Bitetti et al., Reference Di Bitetti, Paviolo and De Angelo2006b, Reference Di Bitetti, De Angelo, Paviolo and Di Blanco2008a).
The slightly higher jaguar density recorded in the 2006 survey at Iguazú may be related to the inclusion of San Jorge Forest Reserve (c. 25% of the sampled area), where six of the eleven adults photographed were recorded. This, and the fact that San Jorge is located in a strategic area serving as a connection between Iguazú National Park and Urugua-í Provincial Park, (Fig. 1b) suggest that this is an important area for jaguar conservation.
It was previously assumed that Crawshaw's density estimates for Iguazú were representative of the whole Green Corridor (Eizirik et al., Reference Eizirik, Indrusiak, Johnson, Medellín, Equihua, Chetkiewicz, Crawshaw, Rabinowitz and Redford2002). For Urugua-í and Yabotí we only have conservative density estimates and there is no previous information to ascertain whether there has been a population decline in these areas as well. However, the fact that we photographed only one jaguar at each site, despite the long duration of the surveys and the large areas studied, the difficulty of finding jaguar tracks, and the low jaguar recording rate compared to similar studies (Table 1; Maffei et al., Reference Maffei, Cuéllar and Noss2004) indicate that jaguar densities are currently extremely low in these areas.
Low availability of prey could have affected this population. A good prey base is essential for the maintenance of healthy jaguar populations (Hoogesteijn & Mondolfi, Reference Hoogesteijn and Mondolfi1992; Crawshaw, Reference Crawshaw1995). In Misiones poaching is common and can reduce prey availability (Paviolo, Reference Paviolo2002). Our camera trap records of collared peccaries Pecari tajacu, tapirs Tapirus terrestris and red brocket deers Mazama americana were also fewer in Urugua-í and Yabotí (with high hunting pressure) than in Iguazú (Di Bitetti et al. Reference Di Bitetti, Paviolo, Ferrari, De Angelo and Di Blanco2008b; A. Paviolo et al., unpubl. data). Densities of other large carnivores such as pumas and ocelots are also lower at Yabotí and Urugua-í (Di Bitetti et al., Reference Di Bitetti, Paviolo and De Angelo2006b, 2008a; A. Paviolo et al., unpubl. data), suggesting they are probably affected by similar factors.
In the northern part of the Green Corridor (including Iguazú and Urugua-í), white-lipped peccaries Tayassu pecari have declined. In the early 1990s they were abundant and comprised the most important prey item in the jaguar's diet (Crawshaw, Reference Crawshaw1995). This species has not been recorded since 2000 in the Iguaçú National Park of Brazil (A. Ricieri, pers. comm.). We did not record it in our survey at Urugua-í and it was rarely recorded during the two surveys at Iguazú National Park, and in unusually small herds (A. Paviolo et al., unpubl. data). The scarcity of this major prey species could have affected jaguars in this area but does not explain the low density of jaguars at Yabotí, where we observed and recorded white-lipped peccaries in large herds.
Even though jaguars are protected by law in Argentina and Brazil they are regularly killed. Jaguar poaching was an important source of mortality in the Iguaçu National Park (Crawshaw, Reference Crawshaw1995), and jaguars are also killed because they occasionally prey on domestic animals (Schiaffino et al., Reference Schiaffino, Malmierca, Perovic, Medellín, Equihua, Chetkiewicz, Crawshaw, Rabinowitz and Redford2002) and because people consider them dangerous (Conforti & Azevedo, Reference Conforti and Azevedo2003). Between 1995 and 2002 at least 70 jaguars were killed in areas neighbouring Iguaçú National Park (Crawshaw, Reference Crawshaw, Medellín, Equihua, Chetkiewicz, Crawshaw, Rabinowitz and Redford2002), and during the last 10 years at least 47 were killed in Northern Misiones (A. Paviolo, unpubl. data).
The conversion of forests into other land uses is an ongoing process that reduces the availability of adequate habitat for jaguars, fragments forest and facilitates poaching in areas that were previously relatively inaccessible. The decline of the jaguar population of the Green Corridor, estimated to number several hundreds c. 15 years ago (Eizirik et al., Reference Eizirik, Indrusiak, Johnson, Medellín, Equihua, Chetkiewicz, Crawshaw, Rabinowitz and Redford2002) but now comprising < 60 adult animals, may have resulted from the interaction of these factors. Because this population is at the southernmost limit of the species’ range its conservation has special relevance. Its disappearance would be one of the final steps towards the extinction of the species in Argentina, where other jaguar populations are also threatened (Altrichter et al., Reference Altrichter, Boaglio and Perovic2006; Di Bitetti et al., Reference Di Bitetti, De Angelo, Paviolo, Schiaffino, Perovic, Corchera and Brown2006a).
However, the population of jaguars in the Upper Paraná Atlantic Forest is still that with the greatest potential for long-term persistence in the Atlantic Forest, despite its relatively small size. To mitigate the impact of the main threats and allow the jaguar population to recover we recommend five actions: (1) Halt the killing of jaguars and their prey, by reinforcing anti-poaching measures both inside and outside protected areas. (2) Implement the existing protected areas and create new ones in strategic locations to improve connectivity between forest areas, thus conserving important habitat and limiting access for poachers. (3) Reduce conflict between cattle ranchers and jaguars by compensating ranchers for losses but also demanding better management practices and penalizing those who kill jaguar. (4) Change human perceptions of the danger from jaguars and inform the public about the species' conservation status using campaigns and environmental education programmes (an example of which is Campaña Yaguareté, Reference Campaña2007, an education campaign recently launched by local NGOs and governmental agencies). (5) Coordinate joint conservation efforts between government institutions in Argentina and Brazil and NGOs (Paviolo et al., Reference Paviolo, De Angelo, Di Blanco, Ferrari, Di Bitetti and Kasper2006). We are now working in partnership with these institutions to develop a recovery plan for the jaguar population of the Green Corridor (Chalukian et al., Reference Chalukian, Schiaffino, Sestello, Guerra, Moschione and Paviolo2006).
Acknowledgements
We are grateful to all the volunteers and park rangers who helped us with field work. We acknowledge the support and permits provided by the Ministry of Ecology, Natural Resources and Tourism of Misiones province (MERNRT) and the National Parks Administration of Argentina. We are grateful to Fundación Vida Silvestre Argentina and the property owners for their support and permission to conduct this work. Financial support was provided by CONICET, Fundación Vida Silvestre Argentina, WWF–USA, WWF–International, WWF–Switzerland, Lincoln Park Zoo, Fundación Antorchas, the Wildlife Conservation Society, Idea Wild, the Rufford Foundation, MERNRT, and the Eden Project through a grant from the Darwin Initiative. We also thank Lía Montti, Andy Noss, Cristina de De Angelo, Peter Crawshaw and an anonymous reviewer for help and comments on the manuscript.
Biographical sketches
Agustín Paviolo has been working on research related to jaguar conservation in the Atlantic Forest since 2001. This research is focused on the factors that affect the abundance of jaguar, puma and their prey, and their conservation in the Atlantic Forest. Carlos Daniel De Angelo conducts research on jaguar distribution and population genetics in relation to Atlantic Forest landscape fragmentation. Yamil Edgardo Di Blanco is participating in various studies on the ecology and conservation of mammals in the north-east of Argentina. Mario Santiago Di Bitetti has been carrying out research in the Atlantic Forest for the last 15 years, focusing on the behaviour, ecology and conservation of primates and felids.







