Introduction
In French Guiana preliminary social surveys (de Thoisy et al., Reference de Thoisy, Spiegelberger, Rousseau, Talvy, Vogel and Vie2003) suggested the Antillean manatee Trichechus manatus manatus was present and seen regularly along the coast and up to 80 km inland, but may have been decreasing in abundance. As the alluvial coastal plain is narrow, the manatee's habitat is naturally restricted to nearby rocky shores and some large rivers, such as the Maroni, Approuague and Oyapock. With their secretive behaviour, relatively undisturbed estuarine habitats and legal protection, the outlook for the future of manatees in French Guiana is positive (Artigas et al., Reference Artigas, Vendeville, Leopold, Guiral and Ternon2003; de Thoisy et al., Reference de Thoisy, Spiegelberger, Rousseau, Talvy, Vogel and Vie2003). Nevertheless, the Antillean manatee is categorized as Endangered on the IUCN Red List (Self-Sullivan & Mignucci-Giannoni, Reference Self-Sullivan and Mignucci-Giannoni2008) because of its restricted area of occurrence and small population size. The manatee faces a number of threats throughout its range, including habitat degradation and loss, collisions with watercraft, entanglement in fishing gear, pollution, human disturbance, natural disasters, and hunting. However, estimates of abundance and habitat preferences are still unavailable on a country-wide basis. Making management decisions based on incorrect categorization of conservation status could accelerate extinction of a species as a result of insufficient management effort (Pool, Reference Pool2013). Hence, a protocol for conducting a long-term and large-scale census of manatees, adapted to local constraints, is required.
Aerial surveys have been used as a tool to assess distribution and abundance of sirenian populations since the 1970s (Reynolds et al., Reference Reynolds, Morales-Vela, Lawler, Edwards, Hines, Reynolds, Aragones, Mignucci-Giannoni and Marmontel2012). However, they are unsuitable for counting manatees inhabiting river basins in French Guiana, and more widely in the Guianas region, because of the difficulty in detecting the animals from an aircraft. Coastal areas of the Guiana Shield, including French Guiana, are particularly rich in sediments and tannins as a result of sediment from the Amazon River (DeMaster et al., Reference DeMaster, Smith, Nelson and Aller1996; Sylvestre et al., Reference Sylvestre, Guiral and Debenay2004), and consequently the waters of rivers and estuaries are turbid. Boat transect surveys using distance sampling have been particularly useful in determining distribution and population size for some riverine cetaceans (Martin et al., Reference Martin, da Silva and Salmon2004; Zhao et al., Reference Zhao, Wang, Turvey, Taylor and Akamatsu2013). However, they assume that the population distribution is random or uniform and visibility of the animals is unobstructed (Aragones et al., Reference Aragones, LaCommare, Kendall, Castelblanco-Martínez, González-Socoloske, Hines, Reynolds, Aragones, Mignucci-Giannoni and Marmontel2012) but the validity of these assumptions for sirenian populations is uncertain (Reynolds et al., Reference Reynolds, Morales-Vela, Lawler, Edwards, Hines, Reynolds, Aragones, Mignucci-Giannoni and Marmontel2012). Boat- and land-based surveys can be useful for studying the distribution, abundance, behaviour, feeding ecology and migration of manatees and dugongs but are difficult to implement over a large area (Aragones et al., Reference Aragones, LaCommare, Kendall, Castelblanco-Martínez, González-Socoloske, Hines, Reynolds, Aragones, Mignucci-Giannoni and Marmontel2012). We provide a quantitative analysis of the distribution and abundance of the Antillean manatee across its known range in the rivers and estuaries of French Guiana, based on data from a range-wide, line transect visual survey combined with a side-scan sonar survey. To our knowledge this is the first attempt to conduct a large-scale survey of a population of manatees in murky rivers, a common habitat type throughout a significant portion of the species’ distribution.
Study area
French Guiana, on the north-east coast of South America, is the largest French overseas department (90,000 km2). The Maroni River marks the political border with Surinam to the west, and the Oyapock River the border with Brazil in the east. French Guiana lies on the Precambrian Guiana Shield, one of the largest blocks of primary tropical forest and a region rich in biodiversity (Hammond, Reference Hammond and Hammond2005). Coastal and marine biodiversity in French Guiana is influenced by the Amazon River, which is a major factor defining the geological structure of estuarine, coastal and shelf marine ecosystems. The coastal waters are turbid, and extensive mudflats occur along the coast (Artigas et al., Reference Artigas, Vendeville, Leopold, Guiral and Ternon2003). The climate is equatorial, characterized by dry and wet seasons, with a maximum variation in mean temperature of 2°C. The dry season is July–November, and the rainy season December–June, interrupted by a variable period of drought during March–April (Lam-Hoai et al., Reference Lam-Hoai, Guiral and Rougier2006). The hydrographic network of French Guiana is characterized by high density as a result of the annual rains and the gently sloping terrain, with maximum flow rate during May–June and minimum generally in November, when the flow rate of the Amazon River is also considerably lower. The river basins vary in size (c. 138–65,830 km2; Tejerina-Garro et al., Reference Tejerina-Garro, de Mérona, Oberdorff and Hugueny2006). The tide is semi-diurnal, with amplitude of up to 2.5 m (spring tides, mesotidal regimes). The coastal habitats are considered to be well preserved, with limited loss and degradation of estuary areas, swamps and mangroves during the last decade (Lefebvre & Verger, Reference Lefebvre and Verger2014).
Methods
Field data acquisition
Our study covered the entire coastal area of French Guiana (c. 330 km of coastline and up to 80 km upstream along the rivers), all at < 200 m altitude, during March–April 2012, October–December 2013 and January–April 2014. The study area was divided into nine units, covering estuaries, beaches and rivers: (1) Coswine, (2) Mana, (3) Iracoubo, (4) Sinnamary, (5) Kourou, (6) Cayenne, (7) Kaw, (8) Approuague and (9) Oyapock. The same field collection methods and approximately the same survey effort were used in each unit. Boat-based line transect surveys were conducted at 4–6 km per hour from a 5 m long boat with a 15 hp outboard motor. Manatees were detected visually or by side-scan sonar (997 SI Combo, Humminbird, Eufaula, USA). Transects were selected strategically according to navigability and the suitability of habitats for manatees. Surveys were conducted in the middle and lower sections of the rivers (< 40 km from the mouth), as exploratory surveys indicated that manatees rarely use the upper parts of the rivers. In narrow rivers (< 50 m width), transects followed the middle and deepest area of the watercourse. For wider rivers the boat was maintained at a distance of c. 20 m from one of the shores.
Once a potential manatee was detected by the sonar, the image was captured, along with the date, time, geographical coordinates, depth and speed. Immediately, the boat was turned back and passed over the location of the record, maintaining the same speed and trajectory, to rule out false identification of inanimate objects as manatees. If the object moved away or changed its position we assumed it was a manatee. In addition to boat transects, fixed-point surveys were conducted by at least four observers scanning 360° around the boat. Fixed-point surveys were also conducted from the shore, from an elevated vantage point such as a hill or a rock. Indirect signs of manatee presence, such as signs of feeding or excrement, were also recorded. Typical signs of manatee feeding on leaves and stalks in macrophyte communities are easily distinguishable by experienced observers.
Data analysis
The images recorded using side-scan sonar were analysed a posteriori to detect manatees. The images selected (on the basis of shape, size and position) were then organized and placed in a catalogue. A blind, online peer-review system was created, and a number of international experts in the use of side-scan sonar imaging in manatee research were invited to participate. The researchers were asked to analyse and score each photograph 0–5 according to the criteria outlined in Table 1. Photographs with a mean score of ≤ 3 were not considered to be evidence of manatee presence and were discarded from the analysis.
Table 1 Criteria used to define confidence values for photographs captured using side-scan sonar as evidence of manatee Trichechus manatus manatus presence.

We calculated the encounter rate in each unit by counting the total number of manatees detected either visually or using side-scan sonar, and dividing by the total survey effort (length in km) in the unit. As both side-scan sonar and visual surveys are likely to miss manatees, we defined a combined encounter rate to represent the minimum encounter rates along the survey transect, based on the highest encounter rate at any point along the river from either visual or sonar records (Martin et al., Reference Martin, da Silva and Salmon2004; Zhao et al., Reference Zhao, Wang, Turvey, Taylor and Akamatsu2013). In the case of the fixed-point surveys the relative abundance was estimated from the number of manatees sighted per hour in each of the nine study units. To provide an indicator of the use of the study area by manatees (Alvarez-Alemán et al., Reference Alvarez-Alemán, Angulo-Valdés, García Alfonso, Powell and Taylor2016), we also calculated a global detection index as the sum of all evidence (presence/absence of feeding tracks, number of sightings and number of sonar detections) divided by the search effort in hours. Multiple pairwise comparisons (Kruskal–Wallis one-way analysis of variance on ranks) were used to investigate the differences in relative abundance indices between units, to detect spatial trends in manatee distribution. Seasonal differences in the global detection index and combined encounter rate were also explored using a normality test (Shapiro–Wilk) and paired t-test or a Mann–Whitney rank sum test if the normality failed.
We developed a species distribution model by using spatial environmental data to make inferences about habitat suitability for manatees. Conceptually, such models are intended to determine and predict components of a species’ ecological niche through space and time (Kearney & Porter, Reference Kearney and Porter2009). We used MaxEnt 3.3.3k (Phillips et al., Reference Phillips, Anderson and Schapire2006) to estimate the likelihood of maximum entropy distribution of each environmental variable across the study area. The algorithm used by MaxEnt has been shown to produce reliable predictions (Elith et al., Reference Elith, Graham, Anderson, Dudík, Ferrier and Guisan2006) and to process presence-only data and small data sets effectively (Wisz et al., Reference Wisz, Hijmans, Li, Peterson, Graham and Guisan2008). As the objective was to investigate possible changes in distribution between habitats according to season, two datasets of manatee records (visual and side-scan sonar detections) were considered in the analysis: a first set to explore habitat use during the wet season, including the 2012 data (30 March–12 April, n = 10) and the 2014 data (27 February–14 April, n = 34), and a second set to explore habitat use during the dry season using the 2014 data (8–30 October, n = 32). The following predictive environmental data were used in a preliminary assessment of the variables of importance: elevation (digital elevation model acquired by the shuttle radar topography mission); habitat categorization (mangroves, high forests, flooded forests and swamps) derived from habitat mapping (Guitet et al., Reference Guitet, Pélissier, Brunaux, Jaouen and Sabatier2015); and the density of the freshwater network, obtained from digital maps. The species occurrence and the prediction of more suitable habitats were found to rely on elevation, mangroves, high forests and flooded forests. The two remaining variables (swamps and the freshwater network) did not contribute to the distribution. The model was run with a convergence threshold of 10−5, a maximum of 1,000 iterations, and linear/quadratic regularization. Cross-validation is important in testing model performance, especially in situations where it is hazardous, costly or impossible to collect additional samples (Li et al., Reference Li, Du and Guo2015). The 20-fold cross-validation method and area under the receiver operating characteristic curve (AUC) method were used to assess the accuracy of the MaxEnt model. The AUC index was used as a measurement of the discriminatory capacity of classification models, which is independent of the choice of threshold (Jiménez-Valverde, Reference Jiménez-Valverde2012).
Results
We completed 56 days of fieldwork, with an effective sampling time of 248.05 hours, 84.82% devoted to boat surveys (n = 124) and 15.17% to fixed-point surveys (n = 52). The duration of boat surveys was 5–390 minutes (mean = 81.09 minutes); surveys from fixed points lasted 10–120 minutes (mean = 48.26 minutes). The boat transects using side-scan sonar covered 1,129.35 km at a mean vessel speed of 5.71 km hour–1, and the mean sonar range was 19.6 m. The mean depth of the surveyed area was 3.78 m (range 1–18.44 m). Fifty-nine instances of manatee sign were recorded in total, eight by fixed-point surveys and 51 by boat-based surveys (Fig. 1, Table 2).
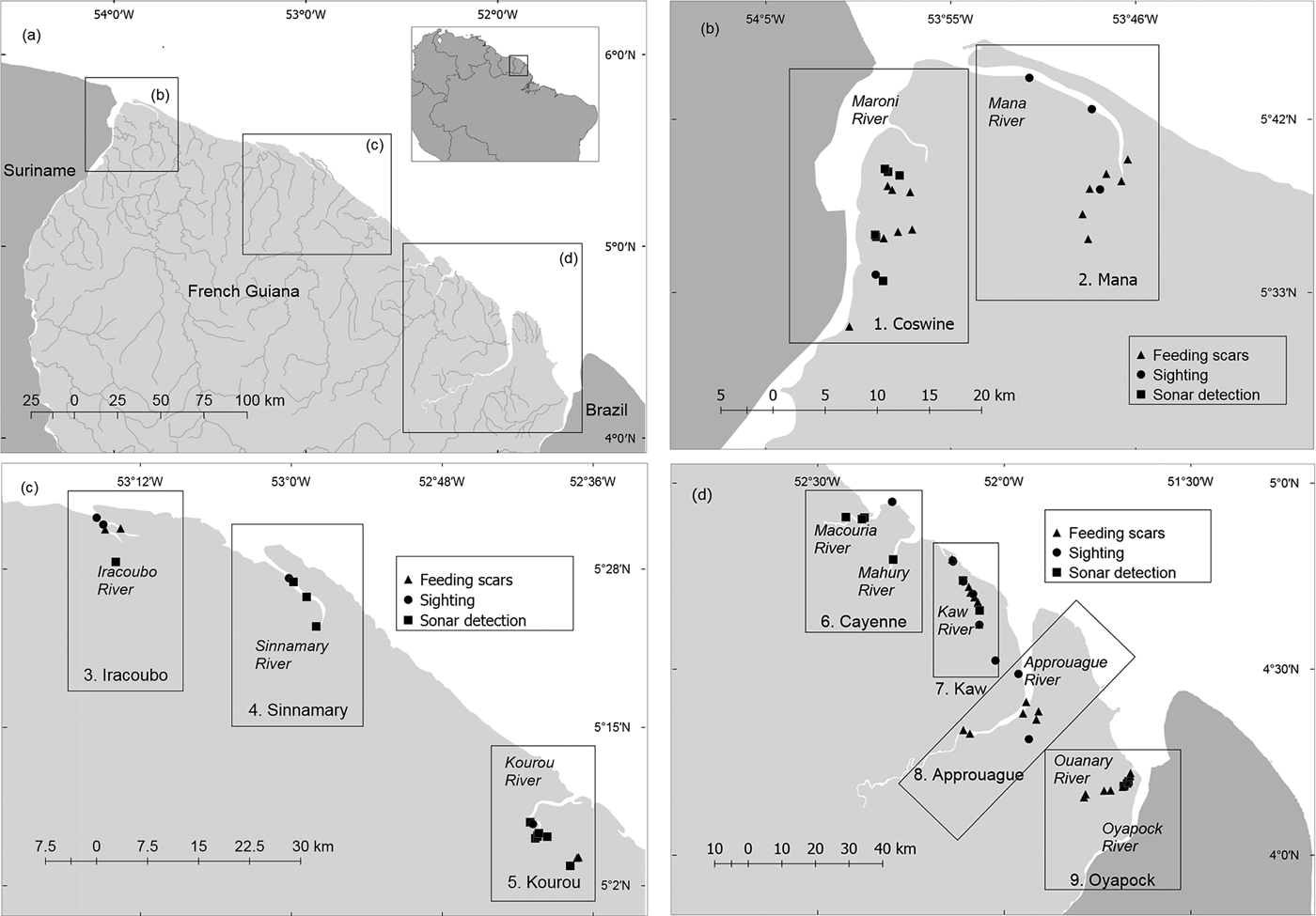
Fig. 1 (a) Distribution and abundance of Antillean manatees Trichechus manatus manatus in French Guiana. (b−d) Distribution of evidences of manatee presence in nine survey units: 1, Coswine; 2, Mana; 3, Iracoubo; 4, Sinnamary; 5, Kourou; 6, Cayenne; 7, Kaw; 8, Approuague; 9, Oyapock.
Table 2 Records of manatee presence in nine study units in French Guiana (Fig. 1), obtained by direct sightings, side-scan sonar, and the presence or absence of feeding scars.

Fifteen direct sightings of manatees were recorded, eight during boat surveys and seven during fixed-point surveys. All were of solitary individuals except one sighting of two individuals in the Kaw River, probably a mother–calf dyad. During the boat transects 1,235 photographs were captured by the sonar, of which 91 were considered to be suitable candidates for the blind peer-review analysis. Six experts accepted our invitation to score the catalogue online. All except one (KAG, who has used this method mostly in shallow swamps) had experience of using side-scan sonar in habitats similar to those found in French Guiana. After discussing the scores provided by the experts, and based on our own field experience, we selected 28 photographs as evidence of manatee presence (Plate 1). Both sightings and detections by sonar were confirmed in most cases by the observation of typical feeding tracks of manatees. Riparian vegetation with typical signs of consumption by manatees was observed during 34 boat surveys, and consisted of patches of aquatic macrophytes such as Mucu-mucu (Crinum sp.) and Gramineae (Echinochloa polystachya). No manatees were detected either by sonar or visually along 73% of the surveyed length, or during 66% of the survey time (148.8 hours).

Plate 1 Examples of side-scan sonar images selected as positive for Antillean manatees in (a) Kourou, 14 October 2013; (b) Cayenne, 15 October 2013; (c) Sinnamary, 16 October 2013; and (d) Oyapock, 20 October 2013 (Fig. 1). M, manatee; S, shadow.
Manatees were detected in all units, resulting in a mean global detection index of 0.49 ± SD 1.22 records per hour, with the highest values in Coswine (1.12 hour–1), Mana (0.65 hour–1) and Kaw (0.56 hour–1; Fig. 2). Boat surveys produced a combined encounter rate of 0.053 ± SD 0.15 per km. Nevertheless, there was not a statistically significant difference among areas in either combined encounter rate (H = 7.814; df = 8, P = 0.452) or global detection index (H = 9.218; df = 8, P = 0.319). The combined encounter rate was greater during the dry season than during the wet season (0.075 ± SD 0.077 and 0.075 ± SD 0.109 encounters km–1, respectively), with non-normal distribution of the data (Shapiro–Wilk, P < 0.05), but the variation was not sufficient to exclude the possibility that it was a result of chance (t = 0.276; df = 8, P = 0.790). In contrast, the global detection index was lower during the dry season than during the wet season (0.448 ± SD 0.34 and 0.652 ± SD 0.63 records per hour, respectively), with a normal distribution of the data (Shapiro–Wilk, P = 0.170). However, the hypothesis that the mean global detection index is significantly greater for the dry season than for the wet season could not be rejected (P = 0.205).
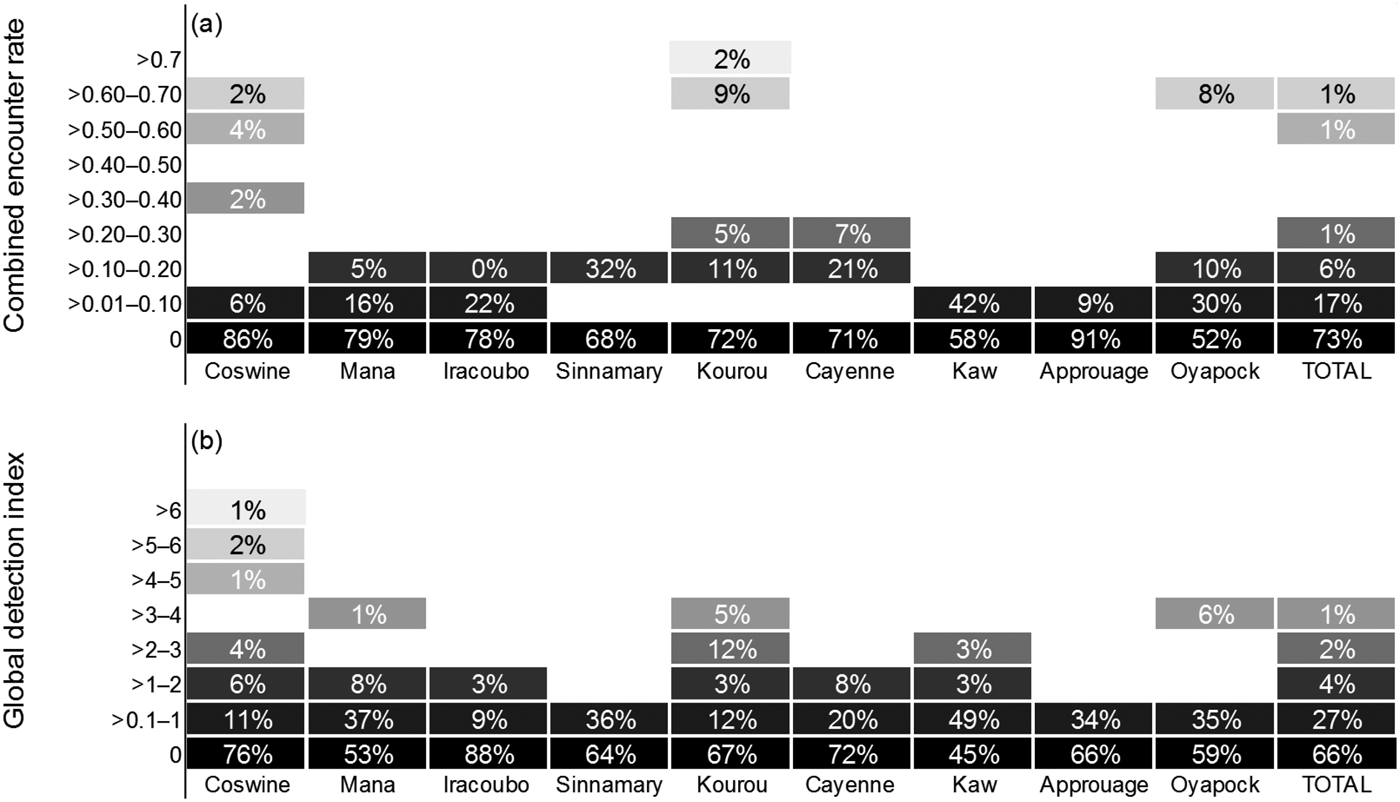
Fig. 2 Relative abundance of Antillean manatees in nine study units in French Guiana (Fig. 1), estimated by (a) combined encounter rate and (b) global detection index. The percentages indicate the proportion of effort in distance (km, combined encounter rate) or time (hours, global detection index) where the index rank was recorded. The colour gradient represents the magnitude of the indices of relative abundance, with the darkest boxes representing the lowest values.
The niche models had comparable relevance for both seasons (AUC = 0.978 and 0.984 for wet and dry seasons, respectively). The percentage contribution of variables to manatee occurrence differed among seasons as follows: mangroves, 12% in wet season, 58% in dry season; flooded forests, 56% in wet season, 12.6% in dry season; dry forests, 21% in wet season, 11% in dry season. These results indicate a higher likelihood of manatee presence in the rivers during the dry season, and a higher likelihood of manatee presence in coastal areas during the wet season (Fig. 3).

Fig. 3 Map of predicted potential distributions of manatees in French Guiana during the dry season, based on four environmental data sets: elevation, mangroves, high forests and flooded forests. Model predictions are illustrated on a spectrum from white (the model predicts absence) to black (the model predicts presence). The results indicate a higher likelihood of manatee presence in the rivers during the dry season (black areas).
Discussion
We aimed to explore and validate methodological and analytical tools for the assessment of seasonal variations in habitat selection and key areas for a secretive mammal in a region of conservation importance. The indices of detection (i.e. combined encounter rate and global detection index) were higher in surveys conducted at Coswine, Kourou and Oyapock, which may be indicative of a higher population density of manatees in those areas. However, the data were not sufficient to demonstrate any significant statistical difference among study units. Also, the type of information obtained was not sufficient to infer whether the differences in the indices are attributable to manatee abundance or to factors affecting manatee detection.
Despite recording evidence of manatee presence in all nine units, detection rates were low throughout the study area, with the highest ranks obtained in only a small proportion of the surveys (Fig. 4); for example, combined encounter rates of > 1.00 per km were recorded for only 0.35% of the total km surveyed, and global detection indices of > 3.00 records per hour were recorded in only 1.43% of the total effort in time. Previous studies conducted blind transect surveys to evaluate the effectiveness of side-scan sonar in various environmental conditions, with preliminary detections of 81 and 93% of the manatees present in the study areas in Florida and Mexico, respectively (Gonzalez-Socoloske et al., Reference Gonzalez-Socoloske, Olivera-Gomez and Ford2009). River basins in French Guiana have similar characteristics to those of Tabasco (Mexico). We detected manatees 19 times with the sonar, and if we assume a detection rate of 93% we estimate the total number of manatees present was 20 individuals. This suggests the manatee population in French Guiana is small compared to other populations. Nonetheless it may be argued, based on interviews and on the availability of extensive high-quality habitat, that manatees are relatively abundant and well conserved in French Guiana, and we suggest that manatee detection rates are lower in French Guiana than in other areas. Our detection capabilities were probably hampered by the manatee's elusive nature, and the noise of the engine is likely to have disturbed manatees in the vicinity of the boat, causing them to flee rapidly from the capture area of the side-scan sonar. There is evidence that manatees are capable of localizing the sounds produced by boats (Colbert-Luke et al., Reference Colbert-Luke, Gaspard, Reep, Bauer, Dziuk, Cardwell and Mann2015), and of reacting to them; for example, disruption of a manatee's call behaviour has been demonstrated at boat–manatee distances of < 25 m (Rivera-Chavarría et al., Reference Rivera-Chavarría, Castro and Camacho2015). As a boat approaches, manatees respond by increasing their swim speed and orienting towards deeper waters (Nowacek et al., Reference Nowacek, Wells, Owen, Speakman, Flamm and Nowacek2004). This bias is likely to be augmented in narrow rivers, where manatees will move downstream or upstream (i.e. not alongside the boat), beyond the capture range of the side-scan sonar. Corroboration of sonar captures in situ was not possible: we failed to visually confirm any of the images captured by the sonar. In French Guiana manatee habitats consist mostly of meandering rivers, and therefore the visible area of the water surface is limited, making it difficult to observe signs of manatees breathing far from the vessel. Also, the waters are generally turbid, making it impossible to visually detect manatees that are underwater.

Fig. 4 Percentage of the survey effort in (a) distance and (b) time, with cumulative number of records of manatee presence in French Guiana (Fig. 1). The combined encounter rate (a) combines only visual and side-scan sonar detections from boat surveys, whereas the global detection index (b) considers all types of evidence: sightings (from boat and fixed-point surveys), side-scan sonar detections, and feeding scars.
The main limiting factor in the use of side-scan sonar is its unknown detection rate (Gonzalez-Socoloske & Olivera-Gomez, Reference Gonzalez-Socoloske and Olivera-Gomez2012). The detection capability can be affected by weather, boat speed, depth, current speed and direction, type of environment, and observer experience. The field biologists who participated in the boat surveys and in the manipulation of the side-scan sonar had various levels of experience and skill. Finally, the data were collected over a 7-month period, with no quantified variations in general hydro-climatic conditions (e.g. depth, which may affect manatee detection). As most of these factors cannot be controlled and are still unknown or poorly understood, it is impossible to correct the error resulting from detectability conditions in the field.
Another factor to take into account when using side-scan sonar is the subjectivity of recognizing manatees in sonar images. Following the precautionary principle, we tried to avoid or reduce overestimation of manatee numbers. However, our method was still subjective and limited by a number of human factors. Errors in image interpretation may include mistaking an object or another large animal for a manatee. Species of megafauna inhabiting watercourses and estuaries in French Guiana include the torche catfish Brachyplatystoma filamentosum (Le Bail et al., Reference Le Bail, Covain, Jégu, Fisch-Muller, Vigouroux and Keith2012), the black caiman Melanosuchus niger (de Thoisy et al., Reference de Thoisy, Hrbek, Farias, Vasconcelos and Lavergne2006), the spectacled caiman Caiman crocodilus (Vasconcelos et al., Reference Vasconcelos, Hrbek, da Silveira, de Thoisy, Marioni and Farias2006), the giant otter Pteronura brasiliensis, the neotropical river otter Lontra longicaudis, and some species of turtles (e.g. Podocnemis expansa). The giant otter has not been reported in habitats used by manatees (Huguin & de Thoisy, Reference Huguin and de Thoisy2016). Fishes with soft bodies, such as catfishes, may absorb too much of the sonar signal to be detected. Larger and more reflective (hard-bodied) fishes, and reptiles such as crocodiles and turtles, are more likely to be imaged by side-scan sonar (Flowers & Hightower, Reference Flowers and Hightower2013). Nevertheless, in general terms, manatees produce a signature shadow that trees, fishes, reptiles, rocks and branches do not; however, field experience and a trained eye are needed to interpret the shape and shadow correctly. Manatees are identified in sonar images based on the following criteria: the manatee's unique signature peanut shape, the paddle shape of the tail, a small head and flippers, and the signature shadow (Brice, Reference Brice2014). Reptiles are also distinctive to a trained observer (Davy & Fenton, Reference Davy and Fenton2013); for example, a crocodile creates an elongated shape with an extended, narrow snout. The exact length of an animal detected by sonar cannot be determined because the image is influenced by body position, vessel speed and water-depth distortions. However, the approximate length of a manatee's acoustic response compared to other objects and the lateral range scale can be used as a size indicator (Brice, Reference Brice2014). Identification was facilitated by the fact that the manatee was the largest completely aquatic vertebrate in the surveyed system.
Comparison of relative abundance indices (combined encounter rate and global detection index) indicated that manatee surveys in turbid rivers are more efficient and productive when several methods and evidence sources are combined (Fig. 4). As none of the methods are infallible, collecting multiple types of evidence increases confidence in the presence/absence data. Although we couldn't confirm any visual detections with images from the side-scan sonar (or vice versa), previous research has validated the use of side-scan sonar to detect manatees in turbid waters (Gonzalez-Socoloske et al., Reference Gonzalez-Socoloske, Olivera-Gomez and Ford2009; Gonzalez-Socoloske & Olivera-Gomez, Reference Gonzalez-Socoloske and Olivera-Gomez2012; Arévalo-González et al., Reference Arévalo-González, Castelblanco-Martínez, Sánchez-Palomino, López-Arévalo and Marmontel2014; Brice, Reference Brice2014).
Seasonality in habitat use
Although the seasonal variation in combined encounter rates and global detection indices was not statistically significant, our niche modelling predicted a greater likelihood of manatee presence in rivers during the dry season, whereas during the wet season manatees seem to prefer coastal areas, as noticed also by local people (B. de Thoisy, pers. obs.). Similar seasonality in habitat preference has been reported for coastal manatees in Belize, where there was found to be a greater probability of manatee presence in the cay habitat (marine environment) than in rivers (freshwater) during the wet season (Auil-Gomez, Reference Auil-Gomez2004). In the tropics manatee distribution is influenced by seasonality (i.e. rainy or dry; Pablo-Rodríguez et al., Reference Pablo-Rodríguez, Olivera-Gómez, Aurioles-Gamboa and Vega-Cendejas2016) rather than fluctuations in water temperature (i.e. summer or winter) as occurs in subtropical regions such as Florida, USA (Irvine, Reference Irvine1983). In tropical freshwater environments where a strong flood pulse occurs (e.g. the Amazon, Orinoco and Usumacinta basins) manatees tend to disperse over larger areas to feed during the rainy season but in the dry season, when the water level drops, they remain confined to smaller areas (Arraut et al., Reference Arraut, Marmontel, Mantovani, Novo, Macdonald and Kenward2010; Castelblanco-Martínez et al., Reference Castelblanco-Martínez, Bermúdez-Romero, Gómez-Camelo, Rosas, Trujillo and Zerda-Ordoñez2009; Pablo-Rodríguez et al., Reference Pablo-Rodríguez, Olivera-Gómez, Aurioles-Gamboa and Vega-Cendejas2016). However, when tropical manatees inhabit both coastal and marine areas, the accessibility of freshwater may become a major limiting factor for their distribution and habitat utilization. There is extensive evidence of the association between occurrence of the Antillean manatee and freshwater sources (e.g. Powell et al., Reference Powell, Belitsky and Rathbun1981; Jiménez, Reference Jiménez2002; Olivera-Gómez & Mellink, Reference Olivera-Gómez and Mellink2005; Castelblanco-Martínez et al., Reference Castelblanco-Martínez, Padilla-Sáldivar, Hernández-Arana, Slone, Reid and Morales-Vela2013a; Landero et al., Reference Landero, de los Ángeles Liceaga-Correa and Morales-Vela2014), and tracking studies have found that Antillean manatees inhabiting salty environments travel several kilometers to the mainland to satisfy their need for freshwater (Castelblanco-Martínez et al., Reference Castelblanco Martínez, Powell, Galves and Auil Gomez2013b). The manatee's need to imbibe freshwater is unusual among marine mammals (MacAvoy et al., Reference MacAvoy, Bacalan, Kazantseva, Rhodes and Kim2015), and although the species apparently has adaptations to survive in salt water (Ortiz et al., Reference Ortiz, Worthy and Byers1999) the use of such mechanisms could have a high energy cost, making it advantageous to remain close to freshwater sources when possible (Olivera-Gómez & Mellink, Reference Olivera-Gómez and Mellink2005). Hence, the temporal variation in freshwater availability may explain the seasonality of habitat selection by manatees in French Guiana, where there is a strong seasonal fluctuation in the relative contributions of marine and continental waters to estuarine waters (e.g. during the dry season the estuarine waters of the Kaw River comprise a mixture of approximately equal amounts of freshwater and seawater (Lam-Hoai et al., Reference Lam-Hoai, Guiral and Rougier2006), and manatees may need to travel upstream to find freshwater to drink). During the rainy season, manatees may remain in coastal areas as the salinity of estuarine waters corresponds to that of fluvial waters originating from the drainage of the river (Lam-Hoai et al., Reference Lam-Hoai, Guiral and Rougier2006).
Conservation
All the localities in French Guiana previously reported by interviews and surveys as being important areas for manatees were confirmed in the field by direct sightings, sonar images and evidence of feeding. The recording of the three types of evidence in all of the basins surveyed indicates that the population is distributed widely across the territory. Additionally, previous interviews (de Thoisy et al., Reference de Thoisy, Spiegelberger, Rousseau, Talvy, Vogel and Vie2003, Castelblanco-Martínez, 2015, unpubl. data) revealed that encounters with manatees in French Guiana are relatively common in comparison with other areas of the species’ distribution, suggesting that the population there is relatively abundant.
Although narrow, given the restricted alluvial coastal plain, suitable habitats for manatees are widely available in French Guiana, and include dense and well-preserved areas of mangrove (Fromard et al., Reference Fromard, Puig, Mougin, Marty, Betoulle and Cadamuro1998). Habitat loss and modification is a significant threat to manatees globally, but it does not seem to be a major concern in French Guiana, where > 90% of the forests are owned by the French state, and are protected against large-scale threats (de Thoisy et al., Reference de Thoisy, Fayad, Clément, Barrioz, Petridou, Poirier and Gond2016). Only two coastal nature reserves, the Amana and Kaw Nature Reserves, provide protection for manatee habitats; however, the Conservatoire du Littoral, a government administrative unit, ensures protection of other coastal areas. Circa 31% of coastal and estuarine area is legally protected, and European Union regulations, national decrees and several laws provide protection outside government-protected areas; for example, the Loi sur l'Eau (Water Law, EU) and the Loi sur la Biodiversity (Biodiversity Law, France) not only address species protection but also incorporate a holistic approach towards the protection of ecological connectivity and networks.
Manatee mortality as a result of entanglement or intentional hunting is apparently low, although awareness of the species needs to be reinforced. However, collision with water vessels may be an increasing threat to manatees, especially in areas with higher human population density, such as Cayenne, and areas frequented by fishers, such as the estuaries. Coswine and Oyapock are located on the international border (with Surinam and Brazil, respectively), where the threats to manatees, including illegal hunting, may be higher, uncontrolled or unknown. Telemetry studies have shown that Antillean manatees can travel up to hundreds of kilometres (Castelblanco-Martínez et al., Reference Castelblanco-Martínez, Padilla-Sáldivar, Hernández-Arana, Slone, Reid and Morales-Vela2013a; Normande et al., Reference Normande, Luna, Malhado, Borges, Viana Junior, Attademo and Ladle2015), and it is likely that at least some individuals are crossing back and forth between nations. There is therefore a need for a wider regional approach to manatee conservation in northern South America. Other threats to manatees, such as contamination caused by gold mining activities, are still poorly understood and merit further consideration.
Assessing manatee abundance and distribution in turbid waters is one of the main obstacles to evaluating the conservation status of this species in the region. Our research has demonstrated advances in the methodology for surveying freshwater and estuarine manatees, particularly in geomorphically and hydraulically complex riverine and estuarine habitats, where distance sampling methods are generally inappropriate. We developed indices of relative abundance that may reflect spatial and temporal trends in population size and facilitate comparisons between locations and, in future, years. Furthermore, we incorporated niche modelling as a novel analytical tool to investigate manatee distributions and seasonality in habitat use. Our results confirmed the presence of manatees in all the rivers and estuaries of French Guiana (suggesting a temporal variation in the use of rivers and coastal areas, probably related to freshwater use and availability), and reinforced the information obtained previously through interviews. As the conservation status of manatees in Amapá State (Brazil) and Surinam is poorly known, Oyapock and Coswine merit particular attention in the development of management plans, and international effort is needed to coordinate actions for manatee preservation. More information is needed regarding the health status, genetic structure and movement of the manatee population in French Guiana to provide a better understanding of its status, abundance and vulnerability and to inform the development of adequate management plans for the species.
Acknowledgements
This study was funded jointly by the French agencies Direction de l'Environnement, de l'Aménagement et du Logement de Guyane and Parc National de La Guadeloupe. The research benefited from technical support from the Marais de Kaw-Roura and Amana Nature Reserves, l'Office National de la Chasse et de la Faune Sauvage and the Protocol Concerning Specially Protected Areas and Wildlife–Regional Activity Centre. We thank Sébastien Barrioz, Billitis Le Guirriec, Sebastién Rives, Régis Gomes, Boris Lerebours and Mathieu Rhoné for their collaboration during field surveys. Katherine Arévalo González, Leon David Olivera, Katie Tripp and three anonymous researchers generously donated valuable time to score and comment on the side-scan sonar images. The comments of two anonymous reviewers helped to improve the article.
Author contributions
DNCM and BdT conceived, designed and managed the surveys. BdT and VdR coordinated the field work. VdR, BdT and DNCM collected data in the field. DNCM performed statistical analysis and mapping. BdT developed the habitat niche model. DNCM, BdT and VdR wrote the article.
Biographical sketches
Delma Nataly Castelblanc-Martínez is a conservation biologist studying aquatic vertebrates in Latin America. Her work focuses on conservation issues, population dynamics, behaviour and ecology of aquatic mammals, particularly manatees. She is Regional Co-chair for South America of the IUCN Sirenian Specialist Group and is currently conducting a long-term megafauna monitoring project in the Caribbean. Virginie dos Reis works for a wildlife conservation NGO in French Guiana and is involved in conservation programmes for marine vertebrates. Benoit de Thoisy has worked on the research and conservation of large vertebrates for 20 years. His main areas of expertise are ecology, population and habitat management, taxonomy and genetics, and awareness issues.









