Introduction
The bush dog Speothos venaticus is a medium-sized (c. 6 kg) Neotropical canid that lives in packs. The species’ biology and population characteristics are poorly understood, and it is one of the least known carnivores in South America (Eisenberg & Redford, Reference Eisenberg and Redford1999; Zuercher et al., Reference Zuercher, Swarmer, Silveira, Carrillo, Sillero-Zubiri, Hoffmann and Macdonald2004; DeMatteo & Loiselle, Reference DeMatteo and Loiselle2008). Most information available on the species in Brazil comes from opportunistic observations (e.g. Peres, Reference Peres1991; Silveira et al., Reference Silveira, Jacomo, Rodrigues and Diniz-Filho1998; Rocha et al., Reference Rocha, Ramalho, Alvarenga, Gräbin and Magnusson2015; Michalski et al., Reference Michalski, Oliveira and Michalski2015a), and it has only recently become the subject of longer-term studies (Lima et al., Reference Lima, Jorge and Dalponte2009, Reference Lima, DeMatteo, Jorge, Jorge, Dalponte, Lima and Klorfine2012, Reference Lima, Jorge, Jorge and Morato2015).
The bush dog is recognized as being a rare carnivore (Zuercher et al., Reference Zuercher, Swarmer, Silveira, Carrillo, Sillero-Zubiri, Hoffmann and Macdonald2004; Oliveira, Reference Oliveira2009; DeMatteo et al., Reference DeMatteo, Michalski and Leite-Pitman2011), and is categorized as Near Threatened on the IUCN Red List (DeMatteo et al., Reference DeMatteo, Michalski and Leite-Pitman2011) and Vulnerable in Brazil (Jorge et al., Reference Jorge, Beisiegel, Lima, Jorge, Leite-Pitman and Paula2013). The Amazon Basin represents the species’ main geographical range and is important for its long-term conservation (e.g. Zuercher et al., Reference Zuercher, Swarmer, Silveira, Carrillo, Sillero-Zubiri, Hoffmann and Macdonald2004; Oliveira, Reference Oliveira2009). Currently, the Amazon has sufficient suitable habitat to maintain a genetically viable population of bush dogs (Oliveira, Reference Oliveira2009).
The Amazon Basin is the largest tract of lowland rainforest, although many areas are under pressure from ongoing habitat loss and fragmentation as a result of human encroachment and development activities, especially along the so-called deforestation arc (Nogueira et al., Reference Nogueira, Yanai, Fonseca and Fearnside2015; Silva et al., Reference Silva, Pereira and Rocha2015). To date, 18.8% of the original forest cover has been lost in the Brazilian Amazon alone (INPE, 2015) and further decline is predicted (e.g. a reduction to 53% of the original cover by 2050; Soares-Filho et al., Reference Soares-Filho, Nepstad, Curran, Cerqueira, Garcia and Ramos2006). The Amazon provides a safe haven for species that range within its boundaries (Rodrigues & Oliveira, Reference Rodrigues, Oliveira, Morato, Rodrigues, Eizirik, Mangini, Azevedo and Marinho-Filho2006), by a combination of extensive tracts of primary forest and a network of protected areas. However, given the current rate of deforestation and the number of existing and proposed hydroelectric dams (Tundisi et al., Reference Tundisi, Goldemberg, Matsumura-Tundisi and Saraiva2014; INPE, 2015) there is likely to be a major shift in the ecological landscape towards many isolated and fragmented habitats; for example, > 10 million ha of forest are expected to be flooded as a result of the construction of hydroelectric dams (Fearnside, Reference Fearnside2006), representing 2% of the total area of the Brazilian Amazon (FAO, 2005), which will contribute directly to widespread extinction of vertebrates (Benchimol & Peres, Reference Benchimol and Peres2015).
The detection and study of cryptic or elusive species has become easier and more reliable with the advent of camera trapping. Although the technique has drawbacks for certain species, such as arboreal or very small species, it has been utilized effectively to study several mammalian carnivores and has proved a reliable source of data for estimating relative abundance and population density (Carbone et al., Reference Carbone, Christie, Conforti, Coulson, Franklin and Ginsberg2001; O'Brien et al., Reference O'Brien, Kinnaird and Wibisono2003; O'Connell et al., Reference O'Connell, Nichols and Karanth2011; Oliveira, Reference Oliveira2011; Kasper et al., Reference Kasper, Mazim, Soares and Oliveira2015). Although the rarity of bush dogs is widely acknowledged, there is no specific quantitative measure of abundance, nor has the abundance of bush dogs been compared with that of other sympatric hypercarnivores. The apparent rarity and distribution of this canid could be a product of competitive interactions with the puma Puma concolor, jaguar Panthera onca, and possibly ocelot Leopardus pardalis (Oliveira & Pereira, Reference Oliveira and Pereira2014), as they share similar prey species (Oliveira, Reference Oliveira, Medellín, Equihua, Chetkiewicz, Crawshaw, Rabinowitz and Redford2002; Zuercher et al., Reference Zuercher, Gipson and Carillo2005; Lima et al., Reference Lima, Jorge and Dalponte2009; Oliveira et al., Reference Oliveira, Tortato, Silveira, Kasper, Mazim, Lucherini, Macdonald and Loveridge2010). To address these issues we estimated the relative abundance of bush dogs in the Brazilian Amazon and compared it with those in other biomes and potential sympatric competitors/predators to assess the long-term conservation and persistence prospects of the species in this area.
Study area
The study focused on four sites in the south-west, central, south-central, and north-east of the Brazilian Amazon (Fig. 1). Three of these were mostly pristine (with slight disturbance; i.e. < 5% alteration by human activity, such as fire or logging) and one was fragmented. The study site at Alto Tarauacá Extractive Reserve, in the south-west, is in an area of open and closed rainforest with alluvial seasonally flooded forest and some degree of disturbance related to the latex extraction industry. The sites surveyed in the Reserve included areas where subsistence hunting had low and high impact (Botelho, Reference Botelho2013). The mid-Tapajós/Amazonia National Park area in central Amazonia is characterized by a humid equatorial climate and hilly terrain with a mosaic of rainforest vegetation types. We used study areas both within and outside the park. The area is dominated by tall terra firme forest (15–30 m) interspersed by areas of lower forest and lianas (10–15 m). There is also an area of seasonally flooded riverine forest (igapó) occupying a narrow strip along the banks of the Tapajós River and its tributaries (George et al., Reference George, Marques, de Vivo, Branch, Gomes and Rodrigues1988). The Amapá National Forest, in the north-east, is a sustainable-use area comprising continuous tropical rainforest vegetation, predominantly never-flooded terra firme forest, with some areas of flooded forest, bamboo and rocky outcrops (Michalski et al., Reference Michalski, Norris, Oliveira and Michalski2015b). The Alta Floresta site is located along the deforestation arc of the Amazon and includes terra firme forest fragments of varying sizes, shapes and degrees of connectivity (Michalski et al., Reference Michalski, Peres and Lake2008).
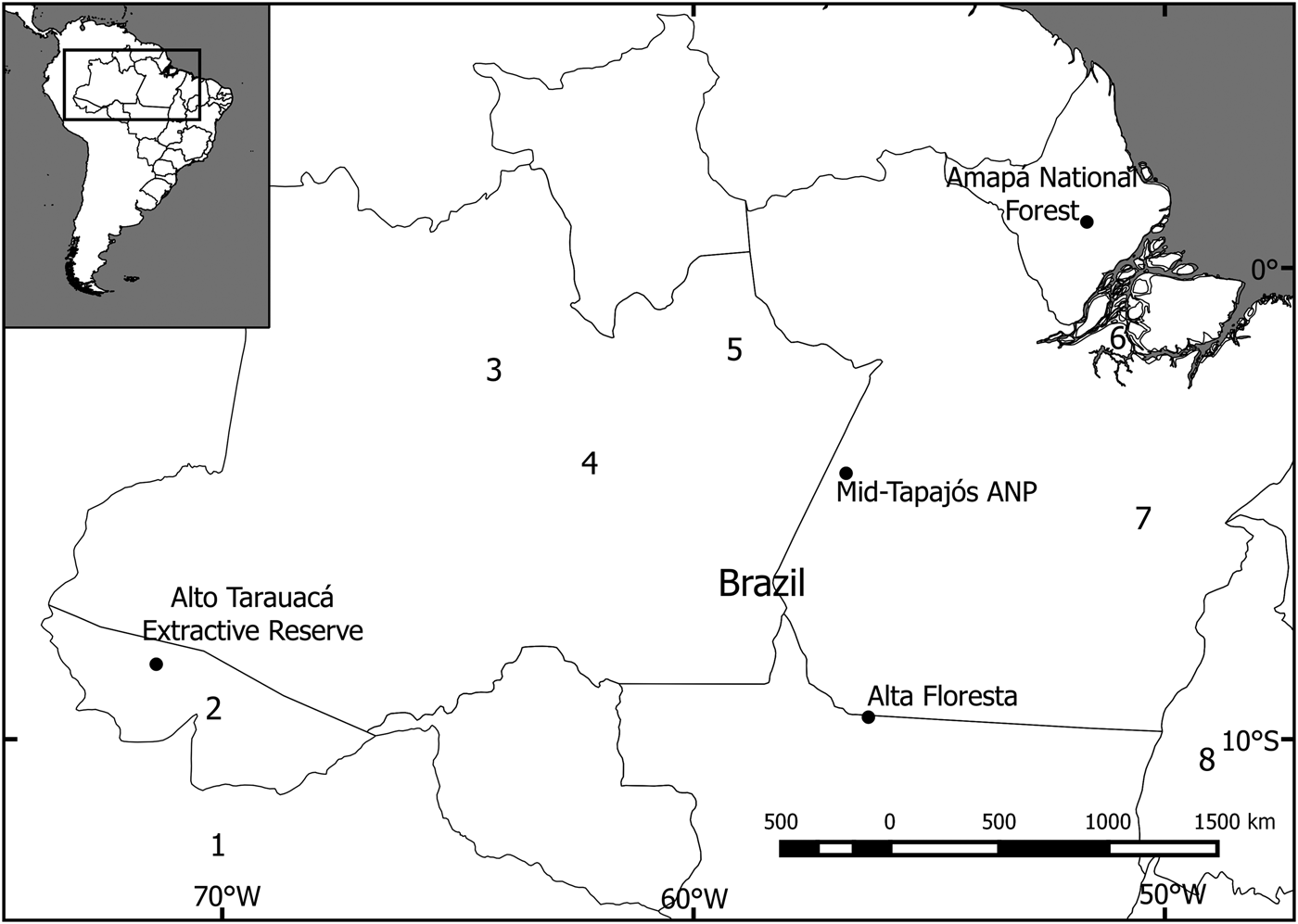
Fig. 1 Locations of the four study sites in the Amazon Basin where camera-trap surveys of the bush dog Speothos venaticus were conducted. Other camera-trap studies have been conducted at (1) Los Amigos Conservation Concession, Peru (Tobler et al., Reference Tobler, Carrillo-Percastegui, Pitman, Mares and Powell2008), (2) Chandless State Park (Borges et al., Reference Borges, Calouro and de Sousa2015), (3) Amanã Sustainable Development Reserve (Rocha et al., Reference Rocha, Ramalho, Alvarenga, Gräbin and Magnusson2015), (4) Piagaçu-Purus Sustainable Development Reserve (Pimenta, Reference Pimenta2012), (5) Balbina Hydroelectric Dam (Benchimol & Peres, Reference Benchimol and Peres2015), (6) Caxiuanã National Forest (Martins et al., Reference Martins, Sanderson and Silva-Júnior2007), (7) Carajás National Forest (Bergallo et al., Reference Bergallo, Carvalho, Reis, Costa, Martins, Castillo, Campos, Hatano and Rolim2012), and (8) Cantão State Park (Negrões et al., Reference Negrões, Revilla, Fonseca, Soares, Jácomo and Silveira2011).
Methods
Defining rarity
A rare species is one that is uncommon, scarce or encountered infrequently (Gaston, Reference Gaston1994). For the purpose of this study we define a rare species as one for which the number of camera-trap records is low, which is an inherent reflection of low density and small population size (Rabinowitz, Reference Rabinowitz and Synge1981). This definition has been used in other studies of mammals (Cofré & Marquet, Reference Cofré and Marquet1999; Yu & Dobson, Reference Yu and Dobson2000; Harcourt et al., Reference Harcourt, Coppeto and Parks2002). We assessed camera-trap data (photographic records) from our study sites (Fig. 1) and for several uncommon and elusive species in the Neotropics (e.g. Martins et al., Reference Martins, Sanderson and Silva-Júnior2007; Tobler et al., Reference Tobler, Carrillo-Percastegui, Pitman, Mares and Powell2008; Negrões et al., Reference Negrões, Revilla, Fonseca, Soares, Jácomo and Silveira2011; Pimenta, Reference Pimenta2012; Botelho, Reference Botelho2013; Oliveira et al., Reference Oliveira, Vieira, Kasper and Trevelin2014; Borges et al., Reference Borges, Calouro and de Sousa2015). Difficult-to-detect species such as the short-eared dog Atelocynus microtis, giant armadillo Priodontes maximus and giant anteater Myrmecophaga tridactyla were all recorded at rates of no more than 0.300 records per 100 trap-days. Thus, we considered species with < 0.300 records per 100 trap-days to be rare.
Bush dog abundance
The location and arrangement of cameras was targeted towards mammalian carnivores. All cameras were spaced 0.5–1 km apart and typically positioned at 20–30 cm height along forest trails, riparian forest/stream banks, and at other sites where carnivores were likely to be detected. The protocol used for camera placement was based on the objective of gathering data on the abundance of carnivores and other mammals, and cameras were placed at optimum distances from the focal area to capture all mammals, and particularly bush dogs (mean shoulder height 25–30 cm; Zuercher et al., Reference Zuercher, Swarmer, Silveira, Carrillo, Sillero-Zubiri, Hoffmann and Macdonald2004). This protocol was also followed to capture data on smaller (< 1 kg) mammals, such as small rodents and marsupials. Thus, we predicted the camera-trap sampling protocol would provide a robust representation of how common or rare carnivores were in these areas. As bush dogs are gregarious, we considered the total number of individuals captured on video or in photographic records to represent relative abundance, reported as individuals per 100 trap-days. Consecutive photographic records of the same species were defined as independent occurrences if the individual(s) could be distinguished unambiguously or if the interval between records was > 30 minutes (O'Brien et al., Reference O'Brien, Kinnaird and Wibisono2003). Bush dog group size was determined from the number of individuals recorded on camera or observed by researchers at the study sites during fieldwork. We assessed bush dog abundance in relation to the abundance of other sympatric hypercarnivores and the bush dog's main prey species, agouti Dasyprocta spp., paca Cuniculus paca and armadillo Dasypus spp. (Zuercher et al., Reference Zuercher, Swarmer, Silveira, Carrillo, Sillero-Zubiri, Hoffmann and Macdonald2004, Reference Zuercher, Gipson and Carillo2005; Lima et al., Reference Lima, Jorge and Dalponte2009).
Analytical procedures
We present data as mean ± standard deviation per sampling unit. We conducted normality tests to determine whether to use parametric or non-parametric statistics. To compare the abundance of bush dogs with that of their potential intraguild predators or competitors we used ANOVA on ranks, and Tukey's test for all pairwise multiple comparisons. We used ANOVA to compare bush dog group size among various vegetative formations, and the Holm–Sidak method for all pairwise multiple comparisons. We applied t-tests to compare bush dog abundance in the Amazon with all other areas in the species’ range as well as abundance in fragmented and non-fragmented sites. We used a Mann–Whitney rank sum test to compare group size from camera-trap data and visual observations, and conducted regression analyses to determine the relationship between bush dog abundance and number of records with trap effort. The significance level was considered to be α = 0.05.
Results
Rarity
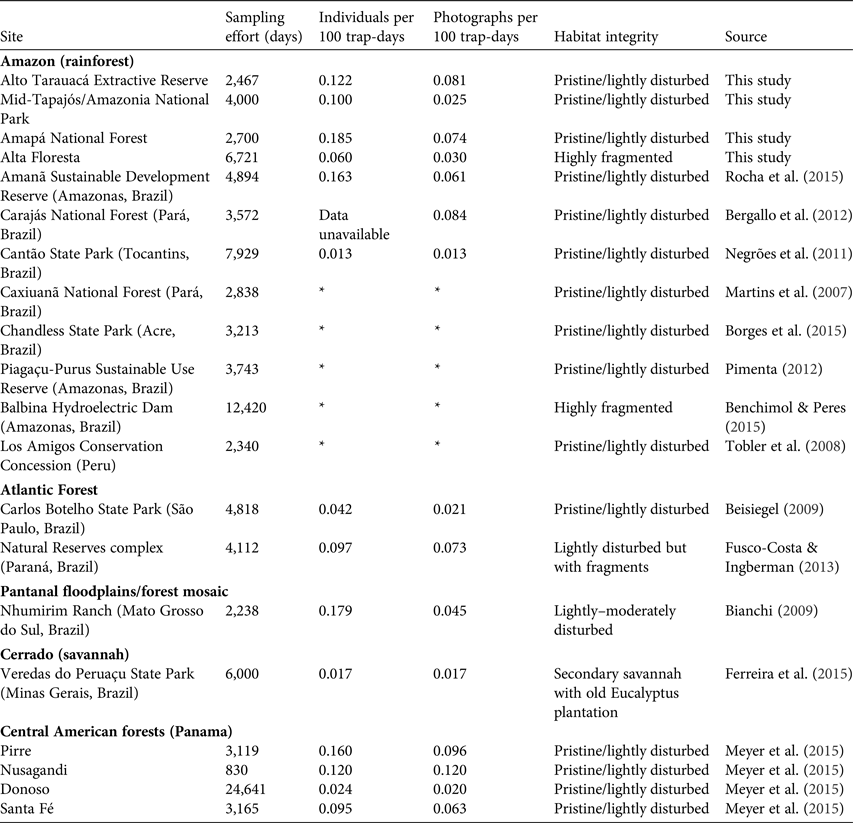
Trapping effort at our four study sites was 15,888 trap-days in total, which yielded a mean relative abundance of 0.117 ± SD 0.052 bush dogs per 100 trap-days (range 0.060–0.185; Table 1). In pristine areas mean abundance was 0.136 ± SD 0.067 individuals per 100 trap-days, more than double that found at the fragmented Alta Floresta site (0.060 individuals per 100 trap-days). The species’ rarity was confirmed by a lack of records in several other similar camera-trap studies across the Amazon, which accounted for an additional 40,949 trap-days (Table 2). Thus, considering the other sites where the focal species was observed (Table 2), the relative abundance of bush dogs in the Amazon was estimated to be 0.013–0.185 individuals per 100 trap-days (mean 0.107 ± SD 0.064; Negrões et al., Reference Negrões, Revilla, Fonseca, Soares, Jácomo and Silveira2011; Bergallo et al., Reference Bergallo, Carvalho, Reis, Costa, Martins, Castillo, Campos, Hatano and Rolim2012; Rocha et al., Reference Rocha, Ramalho, Alvarenga, Gräbin and Magnusson2015; this study). The mean abundance in fragmented and non-fragmented areas across the Amazon Basin was 0.060 and 0.117 ± SD 0.067 individuals per 100 trap-days, respectively. The mean for all camera-trap sites evaluated in the Amazon (including non-detection sites) was 0.058 ± SD 0.072 individuals per 100 trap-days (range 0–0.185; Martins et al., Reference Martins, Sanderson and Silva-Júnior2007; Tobler et al., Reference Tobler, Carrillo-Percastegui, Pitman, Mares and Powell2008; Negrões et al., Reference Negrões, Revilla, Fonseca, Soares, Jácomo and Silveira2011; Pimenta, Reference Pimenta2012; Benchimol & Peres, Reference Benchimol and Peres2015; Borges et al., Reference Borges, Calouro and de Sousa2015; Rocha et al., Reference Rocha, Ramalho, Alvarenga, Gräbin and Magnusson2015; this study). Bush dog abundance in the Amazon was equivalent to that in all other areas outside the Basin (mean 0.092 ± SD 0.061 individuals per 100 trap-days; t = 0.460, df = 12, P = 0.654; Table 2). Additionally, no difference in abundance was found when comparing fragmented/disturbed and non-fragmented areas at any of the study sites (t = 1.351, df = 12, P = 0.202). As abundance was equivalent across all vegetation formations, we conducted regression analyses of bush dog abundance against camera-trap effort across all vegetation types. The expected relative abundance in relation to camera-trap effort was determined as follows: bush dog abundance = 0.130 − (0.00000577effort) (r = 0.557, R 2 = 0.311, F 1, 12 = 5.409, P = 0.038). Considering the number of records, we expected the following: bush dog photographic records = 1.240 + (0.000137effort) (r = 0.6777, R 2 = 0.458, F 1, 12 = 10.160, P = 0.008).
Table 1 Details of our camera trap study to estimate the relative abundance (number of individuals per 100 trap-days) of bush dogs Speothos venaticus and their potential intraguild competitors/predators and prey species at four sites throughout the Brazilian Amazon (Fig. 1).
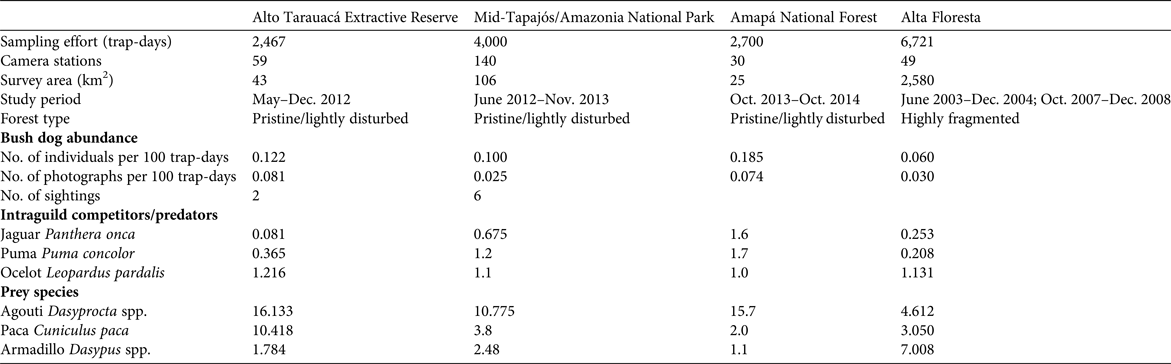
Table 2 Relative abundance of bush dogs recorded in camera-trap studies in major vegetation formations of the Amazon and Atlantic rainforests, the Pantanal floodplains and savannahs in Brazil, and the Central American forests in Panama, with sampling effort, no. of individuals per 100 trap days, habitat integrity, and data source.
* Species present but not detected by camera traps
Group size and activity
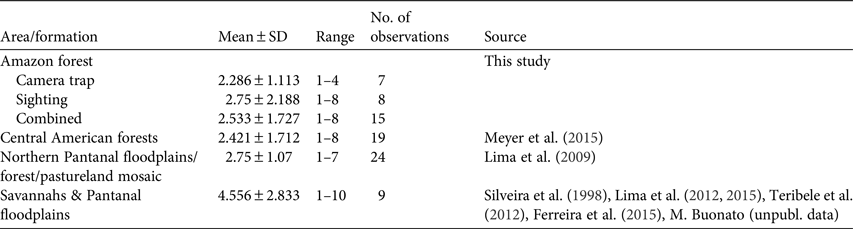
All photographic records and some of the visual observations of bush dogs were from terra firme forest. Bush dogs were mostly recorded along trails (Plate 1) but were also sighted at road crossings, on stream banks and in igapó (flooded forest). The majority of both visual (n = 8, 100%) and camera-trap (n = 7, 71.4%) records occurred during the day. There was no significant difference between group sizes estimated from camera-trap and visual records (T = 56.00, P = 1.00; Fig. 2). We found no significant differences between sizes of bush dog groups in Amazonian forests and in Central America (T = 286.5, P = 0.393; Table 3). However, there were significant differences between forested environments and open formations of the Pantanal floodplains and savannahs (F 3, 67 = 3.397, P = 0.023).
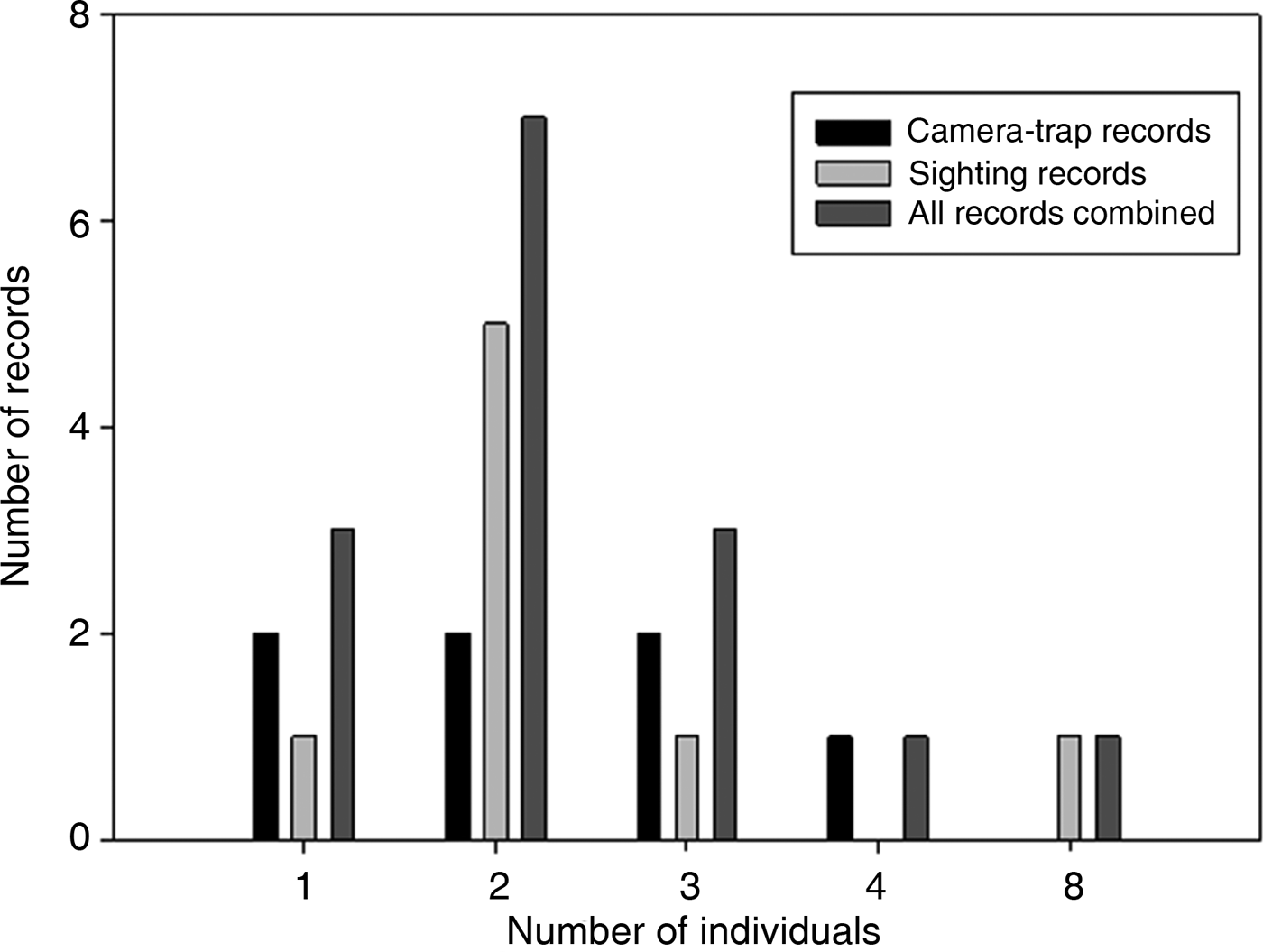
Fig. 2 The total number of bush dogs recorded by camera traps, sightings, and both methods combined, at the four study sites combined (Alto Tarauacá Extractive Reserve, Mid-Tapajós/Amazonia National Park, Amapá National Forest and Alta Floresta; Fig. 1).

Plate 1 A bush dog Speothos venaticus pack at the disturbed and fragmented study site of Alta Floresta, Brazil (Fig. 1). Photograph by Fernanda Michalski.
Table 3 Group sizes of bush dogs recorded in various vegetation formations throughout the species’ range.
Species interactions
The mean relative abundances of jaguars, pumas and ocelots in the study areas were 0.652 ± SD 0.679, 0.868 ± SD 0.705 and 1.112 ± SD 0.089, respectively (Table 1; Supplementary Table S1). All of these species, which are potential intraguild competitors or predators of the bush dog, were significantly more abundant than the bush dog (H = 14.623, df = 3, P = 0.002). Pairwise comparisons of relative abundances between hypercarnivores and bush dogs were also significantly different (P < 0.05). In addition, relative abundances of the main prey species of the bush dog were relatively high at the four study sites (Table 1).
Discussion
Bush dog rarity
The relative abundances of bush dogs in the four study areas were low, corroborating previous observations and conclusions that the species is rare (e.g. Zuercher et al., Reference Zuercher, Swarmer, Silveira, Carrillo, Sillero-Zubiri, Hoffmann and Macdonald2004; Oliveira, Reference Oliveira2009; DeMatteo et al., Reference DeMatteo, Michalski and Leite-Pitman2011; Jorge et al., Reference Jorge, Beisiegel, Lima, Jorge, Leite-Pitman and Paula2013). Although the abundance estimated from camera-trap records at the mid-Tapajós/Amazonia National Park site was lower compared to the other undisturbed areas, the number of visual records at this site was higher than at the other sites. Thus, relative abundance was similar across the study sites, except for the fragmented Alta Floresta area, where it was approximately half that at the other sites. Even with the limited number of records of the bush dog, it is clear that habitat fragmentation is a major conservation issue for this species. Previous studies of the species based on radio tracking have showed a trend of increasing home range with increasing degree of fragmentation of habitat (Lima et al., Reference Lima, DeMatteo, Jorge, Jorge, Dalponte, Lima and Klorfine2012, Reference Lima, Jorge, Jorge and Morato2015; E. Lima, pers. comm.). We found a similar trend at our sites, with higher relative abundances of bush dogs in pristine or only slightly disturbed areas compared with fragmented areas. However, we found no differences when comparing camera-trap records from fragmented/disturbed and non-fragmented areas at any of the study sites. Given the generally low number of records of the species, caution is warranted regarding this issue.
The inherent rarity of bush dogs was also highlighted by the fact that despite a large sampling effort (2,340–12,420 trap-days) the species was not recorded in 42% (5 of 12) of other systematic and robust camera-trap studies across the Amazon (Martins et al., Reference Martins, Sanderson and Silva-Júnior2007; Tobler et al., Reference Tobler, Carrillo-Percastegui, Pitman, Mares and Powell2008; Pimenta, Reference Pimenta2012; Benchimol & Peres, Reference Benchimol and Peres2015; Borges et al., Reference Borges, Calouro and de Sousa2015), most of which were conducted in areas with good habitat integrity. Nevertheless, it is important to highlight that absence of detection by camera-trapping does not prove that the species is absent in an area, only that the low numbers make it difficult to detect. It has been shown that the use of detection dogs is more accurate for locating this rare canid (DeMatteo et al., Reference DeMatteo, Rinas, Sede, Davenport, Argüelles, Lovett and Parker2009).
Relative abundance estimates were from areas where bush dogs were considered to be relatively common (based on visual and/or other evidence). As such, this species is rare even when recorded in greater numbers (Bianchi, Reference Bianchi2009; Meyer et al., Reference Meyer, Moreno, Valdes, Méndez-Carvajal, Brown and Ortega2015; Rocha et al., Reference Rocha, Ramalho, Alvarenga, Gräbin and Magnusson2015; this study). Of all the carnivores that occur in the Amazon Basin only the so-called phantom species, the Amazon weasel Mustela africana and the northern tiger cat Leopardus tigrinus, may be rarer than the bush dog (Oliveira et al., Reference Oliveira, Tortato, Almeida, Campos and Beisiegel2013; Rodrigues, Reference Rodrigues2013).
This general pattern of rarity observed for the bush dog is not restricted to the Amazon Basin but is characteristic of the species throughout its range. Abundance estimates from the Amazon are similar to those from the Atlantic and Central American forests and the Pantanal floodplains and savannahs. Thus, the species is rare in all its habitats, irrespective of the area, biome or method of detection (camera trap, radio telemetry, detection dogs). This rarity is probably related to the species’ inherently low population density, large home range and secretive behaviour (Zuercher et al., Reference Zuercher, Swarmer, Silveira, Carrillo, Sillero-Zubiri, Hoffmann and Macdonald2004; Beisiegel, Reference Beisiegel2009; DeMatteo et al., Reference DeMatteo, Rinas, Sede, Davenport, Argüelles, Lovett and Parker2009; Lima et al., Reference Lima, DeMatteo, Jorge, Jorge, Dalponte, Lima and Klorfine2012, Reference Lima, Jorge, Jorge and Morato2015; Fusco-Costa & Ingberman, Reference Fusco-Costa and Ingberman2013; Ferreira et al., Reference Ferreira, Oliveira, de Paula, Rodrigues and Carmo2015; this study).
Camera-trap records are considered to be a consistent and reliable means of estimating the relative abundance of cryptic mammals (Carbone et al., Reference Carbone, Christie, Conforti, Coulson, Franklin and Ginsberg2001; Goulart et al., Reference Goulart, Cáceres, Graipel, Tortato, Ghizoni and Oliveira-Santos2009; Negrões et al., Reference Negrões, Revilla, Fonseca, Soares, Jácomo and Silveira2011), although a linear relationship between the rate of photographic records and true abundance is assumed, as is a constant detection probability across space and time (O'Connell et al., Reference O'Connell, Nichols and Karanth2011). Thus, it is possible that low abundance estimates reflect low detectability (Lima et al., Reference Lima, DeMatteo, Jorge, Jorge, Dalponte, Lima and Klorfine2012; Ferreira et al., Reference Ferreira, Oliveira, de Paula, Rodrigues and Carmo2015), in which case alternative methods, such as detection dogs, may be more cost-efficient for detecting rare species, including the bush dog (Long et al., Reference Long, Donovan, Mackay, Zielinski and Buzas2007; DeMatteo et al., Reference DeMatteo, Rinas, Sede, Davenport, Argüelles, Lovett and Parker2009). However, detection dogs may not be appropriate for population assessment of all species or in all areas, especially as there are no data on the use of dogs to detect Neotropical carnivores with which to make comparisons. Nevertheless, camera-trapping studies of carnivores, even rare ones, have proven to be effective for detecting and estimating abundance when compared to other techniques, such as telemetry, especially when trapping effort is high (e.g. > 2,000 trap-days; Soisalo & Cavalcanti, Reference Soisalo and Cavalcanti2006; T.G. Oliveira, Reference Oliveira2011, unpubl. data; L.P. Meira et al., unpubl. data; this study). Additionally, bush dog behaviour in the study areas and elsewhere did not suggest avoidance of camera traps or camera locations. The number of records from our study areas also supports this argument. We therefore concur with previous, similar studies and conclude that bush dogs are rare.
In this study we considered any estimates of abundance < 0.300 individuals per 100 trap-days to be indicators of rarity. We are confident that this is an accurate indicator when compared to camera-trap records for other Neotropical carnivores and rare or uncommon Neotropical mammals that were accurately sampled using the same technique (e.g. Tobler et al., Reference Tobler, Carrillo-Percastegui, Pitman, Mares and Powell2008; Negrões et al., Reference Negrões, Revilla, Fonseca, Soares, Jácomo and Silveira2011; Botelho, Reference Botelho2013; Oliveira et al., Reference Oliveira, Vieira, Kasper and Trevelin2014; L.P. Meira et al., unpubl. data).
Group size and activity
All photographic records were from terra firme forest; however, we also recorded sightings and track records in other areas, including at highway crossings, on stream banks and in igapó. Lima et al. (Reference Lima, DeMatteo, Jorge, Jorge, Dalponte, Lima and Klorfine2012, Reference Lima, Jorge, Jorge and Morato2015) reported that radio-tracked groups of bush dogs did not utilize roads, whereas Fusco-Costa & Ingberman (Reference Fusco-Costa and Ingberman2013) observed that they did. In any case, at our study sites cameras were not placed along roads, only on narrow trails. Similar to Rocha et al. (Reference Rocha, Ramalho, Alvarenga, Gräbin and Magnusson2015), the majority of our records suggest that this canid is predominantly diurnal. However, in the savannahs of Mato Grosso state (Brazil) a radio-tracked pack showed higher levels of activity both early in the morning and at night (Lima et al., Reference Lima, DeMatteo, Jorge, Jorge, Dalponte, Lima and Klorfine2012).
Typical groups of bush dogs are estimated to comprise 2–12 individuals, and most observations suggest group sizes of 2–6 (Zuercher et al., Reference Zuercher, Swarmer, Silveira, Carrillo, Sillero-Zubiri, Hoffmann and Macdonald2004). Previous camera-trap studies of the bush dog recorded groups of 1–8 individuals (Beisiegel, Reference Beisiegel2009; Bianchi, Reference Bianchi2009; Negrões et al., Reference Negrões, Revilla, Fonseca, Soares, Jácomo and Silveira2011; Fusco-Costa & Ingberman, Reference Fusco-Costa and Ingberman2013; Meyer et al., Reference Meyer, Moreno, Valdes, Méndez-Carvajal, Brown and Ortega2015; Rocha et al., Reference Rocha, Ramalho, Alvarenga, Gräbin and Magnusson2015), which is similar to our results. Mean group sizes recorded in the Amazon and Panamanian forests were c. 2.5 individuals (Meyer et al., Reference Meyer, Moreno, Valdes, Méndez-Carvajal, Brown and Ortega2015). In a Pantanal floodplain savannah/forest/pastureland mosaic the mean group size (2.75) was only slightly larger than in forested areas (Lima et al., Reference Lima, Jorge and Dalponte2009). However, limited data from other open habitats in seasonally flooded floodplains and savannahs suggest larger and significantly different group sizes than those in forested areas (Lima et al., Reference Lima, DeMatteo, Jorge, Jorge, Dalponte, Lima and Klorfine2012, Reference Lima, Jorge, Jorge and Morato2015; Teribele et al., Reference Teribele, Concone, Godoi, Bianchi, Santos and Mauro2012; Ferreira et al., Reference Ferreira, Oliveira, de Paula, Rodrigues and Carmo2015; Meyer et al., Reference Meyer, Moreno, Valdes, Méndez-Carvajal, Brown and Ortega2015; this study). Differences in group size between savannahs and forests may reflect differences in the availability or accessibility of the bush dog's main prey species between these vegetative formations. The predominantly small group size may be explained in part by the more solitary behaviour of Neotropical hypercarnivores (Oliveira & Pereira, Reference Oliveira and Pereira2014), with the formation of groups being related to the need to subdue larger prey in some habitats (Zuercher et al., Reference Zuercher, Swarmer, Silveira, Carrillo, Sillero-Zubiri, Hoffmann and Macdonald2004, Reference Zuercher, Gipson and Carillo2005).
Species interactions
The abundance of bush dogs at the study sites was low, and up to 10 orders of magnitude lower compared to intraguild competitors and predators. However, the highest abundance estimates for large felids in the study area are for Amapá National Forest, where the highest abundance of bush dogs was also recorded. Conversely, in the Tarauacá Extractive Reserve, where bush dog abundance was almost as high as in Amapá, large felids were uncommon. Although this appears to be contradictory, we suggest that our data can neither confirm nor deny the existence of intraguild interactions, which have been demonstrated for smaller Neotropical felids (Oliveira et al., Reference Oliveira, Tortato, Silveira, Kasper, Mazim, Lucherini, Macdonald and Loveridge2010; Oliveira, Reference Oliveira2011). Negative impacts of dominant competitors on subordinate competitors include decreases in niche breadth, restriction of habitat to less favourable areas, changes in activity patterns, and localized extinction, in addition to being preyed upon. Thus, subordinates tend to have smaller population sizes than dominant competitors (e.g. Miquelle et al., Reference Miquelle, Stephens, Smirnov, Goodrich, Zaumyslova, Myslenkov, Ray, Redford, Steneck and Berger2005; St-Pierre et al., Reference St-Pierre, Ouellet and Crête2006; Saleni et al., Reference Saleni, Gusset, Graf, Szykman, Alters and Somers2007; Oliveira et al., Reference Oliveira, Tortato, Silveira, Kasper, Mazim, Lucherini, Macdonald and Loveridge2010; Oliveira, Reference Oliveira2011). Thus, bush dog rarity may be a result of competitive interactions with jaguars, pumas and perhaps ocelots, as they share a similar prey base (Oliveira, Reference Oliveira, Medellín, Equihua, Chetkiewicz, Crawshaw, Rabinowitz and Redford2002; Zuercher et al., Reference Zuercher, Gipson and Carillo2005; Lima et al., Reference Lima, Jorge and Dalponte2009; Oliveira et al., Reference Oliveira, Tortato, Silveira, Kasper, Mazim, Lucherini, Macdonald and Loveridge2010; Oliveira & Pereira, Reference Oliveira and Pereira2014). Predation by domestic dogs could also be a threat in some areas (Ferreira et al., Reference Ferreira, Oliveira, de Paula, Rodrigues and Carmo2015; E. Lima, pers. comm.).
Relative abundances of the bush dog's main prey items (i.e. agouti, paca and armadillo) were relatively high at all of our study sites. Although prey availability is a defining factor of carnivore abundance, we suggest it may not be the limiting factor determining inherent bush dog rarity, as this would not explain their low abundance at our study sites or elsewhere (Ferreira et al., Reference Ferreira, Oliveira, de Paula, Rodrigues and Carmo2015; this study).
Conservation issues
The expansion of hydroelectric energy projects in the Amazon Basin, where most of the viable populations of bush dogs occur, will continue and possibly accelerate in the future. Current estimates vary from 94 to 151 additional dam projects planned for Amazonia (Tundisi et al., Reference Tundisi, Goldemberg, Matsumura-Tundisi and Saraiva2014). This will introduce large-scale changes to the hydrological cycle, inland connectivity and ecosystem services, which will in turn affect the persistence and maintenance of several species populations. This future scenario for the Amazon Basin in Brazil is of particular concern (Soares-Filho et al., Reference Soares-Filho, Nepstad, Curran, Cerqueira, Garcia and Ramos2006). The population viability assessment conducted for Amazonia National Park showed a 50% probability of extinction of the bush dog in < 100 years if populations become isolated (Godoy et al., Reference Godoy, Desbiez and Miller2015). The overall scenario for this area is predicted to worsen as the population size and carrying capacity decrease as a result of the suppression of flooded riverine forest following the installation and operation of a hydroelectric dam (Godoy et al., Reference Godoy, Desbiez and Miller2015). Simulations indicate that isolation, which has already begun in some places (Nogueira et al., Reference Nogueira, Yanai, Fonseca and Fearnside2015; Silva et al., Reference Silva, Pereira and Rocha2015), reduces the likelihood that even forest reserves of > 10,000 km2 would be adequate to maintain viable populations of bush dogs and to provide the conditions necessary for the long-term conservation of this rare canid.
Conclusions
Extensive tracts of undisturbed forest with low levels of human impact dominated the study areas, except for Alta Floresta, yet even in pristine areas the bush dog is naturally rare and threatened by predation and disease. Sarcoptic mange in particular is one of the main threats to the species in all Brazilian biomes (e.g. Jorge et al., Reference Jorge, Beisiegel, Lima, Jorge, Leite-Pitman and Paula2013; Godoy et al., Reference Godoy, Desbiez and Miller2015). Disease can cause the loss of species from some areas for prolonged periods, as observed for short-eared dogs in Peru (Leite-Pitman & Williams, Reference Leite-Pitman and Williams2011). Diseases affecting wild canids tend to be transmitted from domestic dogs, which are commonly brought into protected and unprotected forested areas by local and indigenous people (Leite-Pitman et al., Reference Leite-Pitman, Nieto, Davenport, Leite Pitman, Pitman and Alvarez2003; Leite-Pitman & Williams, Reference Leite-Pitman and Williams2011). However, it is unlikely that disease alone could pose a simultaneous threat across the entire range of the bush dog, or be the sole cause of its rarity. Rather, the inherent rarity of the bush dog (defined here as < 0.300 individuals per 100 trap-days) appears to be attributable to a combination of competitive pressure and predation (intraguild or otherwise), habitat structure and integrity, and disease, acting synergistically (but not separately) to determine the abundance and degree of rarity in any particular area.
Acknowledgements
We thank CNEC WorleyParsons Engenharia S.A. and Eletrobras, who provided logistical and financial support for the fieldwork in the mid-Tapajós/Amazonia National Park area. Fieldwork there depended on the assistance of many colleagues; we are especially indebted to Fábio Mazim, Gilberto Nascimento, Jean Pierre Santos, and José Bonifácio Soares. We thank Instituto Chico Mendes de Conservação da Biodiversidade and the Federal University of Amapá for logistical support at the Amapá National Forest study site, and the Programa de Pesquisa em Biodiversidade Biodiversity Monitoring Programme for providing the grid system used during field activities there. We thank Cremilson Marques, Érico Kauano and Sueli dos Santos for their assistance during the field work. The United Nations Development Programme (UNDP), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and Fundação de Tecnologia do Estado do Acre funded the work in Acre. Rosenil Oliveira and Cláudia Cunha provided field assistance in Acre. Fundação de Amparo à Pesquisa e ao Desenvolvimemto Científico e Tecnológico do Maranhão (FAPEMA) provided additional support. Nuno Negrões and several anonymous reviewers provided important and insightful comments.
Author contributions
TGO, FM, ALMB and LJM collected and analysed the data, and co-wrote the article with AMC and ALJD.
Biographical sketches
Tadeu de Oliveira’s main research interests are the ecology and conservation of Neotropical carnivores. Fernanda Michalski’s research interests are in conservation biology and the ecological consequences of habitat fragmentation on large and mid-sized vertebrates. André Botelho is a wildlife ecologist and is interested in the ecology and conservation of medium-to-large sized mammals. Lincoln Michalski is a wildlife ecologist whose main research interests are in the ecology and conservation of mammals. Armando Calouro is interested in the effects of anthropogenic perturbations on mammals in the south-western Brazilian Amazon. Arnaud Desbiez’s research focuses on sustainable use of natural resources and species conservation.








