Introduction
Neotropical biodiversity is severely threatened by habitat degradation as a consequence of human population growth, climate change and human-caused fires (Ibisch & Mérida, Reference Ibisch and Mérida2004; Kosydar et al., Reference Kosydar, Rumiz, Conquest and Tewksbury2014; Peñaranda & Simonetti, Reference Peñaranda and Simonetti2015). In Bolivia, the continued expansion of the livestock industry is the main contributor to deforestation (Müller et al., Reference Müller, Müller, Schierhorn, Gerold and Pacheco2012). This is especially true for the diverse ecosystems of the Chiquitano region in eastern Bolivia (Ibisch & Mérida, Reference Ibisch and Mérida2004; Killeen et al., Reference Killeen, Chavez, Pena-Claros, Toledo, Arroyo, Caballero, Pennington, Lewis and Ratter2006; Navarro, Reference Navarro2011). The endemic Chiquitano Dry Forest is the largest block of tropical broad-leaf dry forest in South America (Miles et al., Reference Miles, Newton and DeFries2006; Power et al., Reference Power, Whitney, Mayle, Neves, de Boer and Maclean2016). During 2001–2006, c. 15% of the original extent of the Chiquitano Dry Forest was deforested at a mean rate of 1,080 km2 per year (Killeen et al., Reference Killeen, Chavez, Pena-Claros, Toledo, Arroyo, Caballero, Pennington, Lewis and Ratter2006). In addition, recent widespread human-caused wildfires have destroyed 12% of the Chiquitano Dry Forest, with drastic consequences for biodiversity (Devisscher et al., Reference Devisscher, Anderson, Aragão, Galván and Malhi2016; Romero-Muñoz et al., Reference Romero-Muñoz, Jansen, Nuñez, Almonacid and Kümmerle2019a,Reference Romero-Muñoz, Torres, Noss, Giordano, Quiroga and Thompsonb). Many of its small-sized vertebrate species have only been described recently (Caminer et al., Reference Caminer, Milá, Jansen, Fouquet, Venegas and Chávez2017; Jansen et al., Reference Jansen, Santana, Teixeira and Köhler2019; Pansonato et al., Reference Pansonato, Motta, Cacciali, Haddad, Strüssmann and Jansen2020), a large portion remains unknown (Jansen et al., Reference Jansen, Bloch, Schulze and Pfenninger2011; Gehara et al., Reference Gehara, Crawford, Orrico, Rodriguez, Loetters and Fouquet2014) and few ecological studies have investigated its mammalian fauna (Anderson, Reference Anderson1997; Brooks et al., Reference Brooks, Rojas, Aranibar, Vargas and Tarifa2002). Long-term biodiversity monitoring programmes are scarce in this region, hindering the documentation and understanding of anthropogenic biodiversity loss, such as that resulting from land-use change.
Our study focuses on the jaguar Panthera onca (Plate 1), the Neotropical apex predator. The jaguar is considered a wildlife indicator species (Thornton et al., Reference Thornton, Zeller, Rondinini, Boitani, Crooks and Burdett2016) and suffers significantly from illegal hunting, habitat destruction and forest fragmentation (Wolf & Ripple, Reference Wolf and Ripple2017; Tucker et al., Reference Tucker, Böhning-Gaese, Fagan, Fryxell, Van Moorter and Alberts2018; Romero-Muñoz et al., Reference Romero-Muñoz, Benítez-López, Zurell, Baumann, Camino and Decarre2020). Moreover, poaching of jaguars has intensified as a result of negative attitudes towards the species determined by socioeconomic factors (Caruso et al., Reference Caruso, Perovic, Talamo, Sillero-Zubiri and Altrichter2022), local human–jaguar conflicts (Supplementary Plates 1 & 2; Wallace et al., Reference Wallace, Gomez, Zulia and Rumiz2010) and trafficking of skulls, claws and fangs to satisfy the Asian traditional medicine market (Nuñez & Aliaga-Rossel, Reference Nuñez and Aliaga-Rossel2017; Fraser, Reference Fraser2018). At an ecoregional scale (the Chaco region of Paraguay, Argentina and Bolivia), the jaguar distribution decreased by 33% during 1985–2013, mainly because of illegal hunting and habitat loss (Romero-Muñoz et al., Reference Romero-Muñoz, Benítez-López, Zurell, Baumann, Camino and Decarre2020), and at the national scale the natural vegetation in central-eastern Bolivia decreased by > 40% during 1976–2005 (Zemanova et al., Reference Zemanova, Perotto-Baldivieso, Dickins, Gill, Leonard and Wester2017). As a consequence, the Bolivian jaguar population has declined considerably, from occupying c. 75% to c. 50% of the country (Maffei et al., Reference Maffei, Rumiz, Arispe, Cuellar, Noss, Medellín, de la Torre, Zarza, Chávez and Ceballos2010). In the department of Santa Cruz, deforestation has been identified as a serious threat to the jaguar, and it has been predicted that its habitat (263,000 km2) will be reduced by c. 50% (to 137,000 km2) by 2046 (Maillard et al., Reference Maillard, Angulo, Vides-Almonacid, Rumiz, Vogt and Monroy-Vilchis2020). Thirty-nine connecting corridors of 58,000 km2 between protected areas, involving c. 5,700 cattle ranches, have been previously identified, mainly in the central Chiquitano region (Maillard et al., Reference Maillard, Angulo, Vides-Almonacid, Rumiz, Vogt and Monroy-Vilchis2020). Thus, cattle ranches with suitable habitats should be given more attention in the context of conservation.

Plate 1 Recognition of jaguar Panthera onca individuals based on unique coat colour patterning. All five images are from different capture events in the Chiquitano region of the eastern Bolivian lowlands (Fig. 1) and display the same jaguar individual (female F-03) from different angles. The images are of varying image quality, to demonstrate how we used unique coat patterning to identify the same individual.
The protection of jaguars is of crucial ecological importance as they are a keystone species and play an important role in sustaining balanced ecosystems by regulating prey populations. Camera traps facilitate continual, non-invasive, long-term monitoring of jaguars to measure shifts in population structure or to estimate population sizes (Silver et al., Reference Silver, Ostro, Marsh, Maffei, Noss and Kelly2004). Previous studies indicated that the Chiquitano Dry Forest harbours a substantial but understudied population of jaguars and their prey (Rumiz et al., Reference Rumiz, Fuentes, Rivero, Santiváñez, Cuéllar and Miserendino2002; Arispe et al., Reference Arispe, Rumiz and Noss2007; Venegas et al., Reference Venegas, Rumiz, Angulo and Rivero2010; Polisar et al., Reference Polisar, de Thoisy, Rumiz, Díaz Santos, McNab and Garcia-Anleu2016). Here we report on an ongoing camera-trapping survey established in 2017 (Jansen et al., Reference Jansen, Engler, Blumer, Rumiz, Aramayo and Krone2020). We provide rare insights into a healthy jaguar population in a South American dry forest habitat by characterizing complex life history trajectories and by estimating the density and relative abundance of jaguars in relation to land use. Furthermore, we highlight an alarming rate of deforestation by tracking changes in the forest cover of our study area over a 5-year period. The scientific evidence we have gathered could be used to discourage further deforestation, to preserve the faunal and floral diversity of the region.
Study area
Our study area (Fig. 1) covers 133 km2 and comprises nine cattle ranches in the Chiquitano region of the eastern Bolivian lowlands. Approximately 70% of the area was covered by dense forest vegetation at the beginning of the study period in 2017. Extensive livestock farming is practised in open areas but no commercial crops are grown. The area is at an altitude of 500 m and falls within a climatic and biogeographical transition zone between the Amazon rainforest, the Gran Chaco Dry Forest and the Cerrado savannah of Brazil. Temperatures vary marginally throughout the year, with a mean daily temperature of 24.4 °C (Killeen et al., Reference Killeen, Chavez, Pena-Claros, Toledo, Arroyo, Caballero, Pennington, Lewis and Ratter2006). Mean annual precipitation is c. 1,200 mm (Schulze et al., Reference Schulze, Jansen and Köhler2009), with a dry season during July–November (Killeen et al., Reference Killeen, Chavez, Pena-Claros, Toledo, Arroyo, Caballero, Pennington, Lewis and Ratter2006). The Chiquitano Dry Forest is the primary vegetation type and is characterized by relatively open forests with semi-deciduous trees interspersed with grasses and shrubs of the woody savannah (Killeen et al., Reference Killeen, Chavez, Pena-Claros, Toledo, Arroyo, Caballero, Pennington, Lewis and Ratter2006). In September 2020, a project aiming to convert c. 15 km2 of partially protected private forest to pasture was initiated in the core study area and land-use change is thus likely to increase.

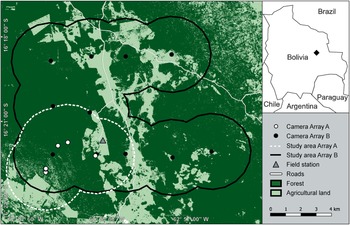
Fig. 1 Location of the study area in the Chiquitano region of the eastern Bolivian lowlands, showing the layout of the two camera-trap arrays used during 2017–2019. A buffer of 2.5 km around each camera station was used to delineate study plot boundaries. The spacing between camera stations in Array B was c. 2.5 km, to account for the home range size of the jaguar Panthera onca. We derived the land use classification from August 2019 imagery.
Methods
Study design
We conducted two camera-trap surveys during March 2017–December 2019 using two partially overlapping camera-trap arrays (Fig. 1). Array A, comprising 13 stations, was active from March 2017 (Jansen et al., Reference Jansen, Engler, Blumer, Rumiz, Aramayo and Krone2020) to December 2019. Cameras in Array A were placed opportunistically across a 40 km2 area along dirt roads, stream beds and game trails to increase the probability of detecting species (Fig. 1). We set the cameras in Array A mostly to photo mode, or to video mode where the frequency of jaguar captures was high, and cameras collected data from a total of 16,104 trap-nights. We set up Array B, comprising 11 stations (each with paired cameras), in March 2019, and we considered data collected until December 2019 in this study. We laid out the cameras in Array B as a symmetric grid covering 133 km2 (Fig. 1). We set the spacing between camera stations in Array B to c. 2.5 km to account for the home range size of jaguars (Maffei et al., Reference Maffei, Noss, Silver, Kelly, O'Connell, Nichols and Karanth2011). We set the camera traps in Array B to photo mode with a burst of three images per trigger event and a minimum delay of 5 s between events. Cameras in Array B were active for a total of 4,247 trap-nights. We used a buffer of 2.5 km around each camera station to delineate study plot boundaries in Array A and Array B (Fig. 1).
Camera models used were Bushnell Trophy Camera Brown Model 119437 (n = 26; Bushnell, Overland Park, USA), Reconyx XR6 UltraFire (n = 3; Reconyx, Holmen, USA) and Cuddeback G-series Double Barrel Strobe (n = 18; Cuddeback, Green Bay, USA). We placed all camera stations in suitable microsites (e.g. animal trails) and attached them to trees 30–40 cm above the ground. We visited each camera trap approximately every 2 weeks to change the batteries and download data. Not all camera traps were active consecutively because of occasional battery problems or failures caused by humidity. For camera station operation times see Supplementary Figs 1 & 2.
We carried out all analyses in R 3.6.1 (R Core Team, 2022) if not specified otherwise. All software and associated version numbers, with references, are listed in Supplementary Table 1.
Image processing
To integrate videos into the processing workflow, we extracted three frames per video (t = 0 seconds, t = 2 seconds, t = 4 seconds) using a python script (ExtractFramesFromVideo.py, settings —frameTimeLimit 4 -f 0.51). We uploaded all images and extracted frames to Labelbox (Labelbox, 2021), a web-based labelling platform used to create training datasets for machine learning applications. We involved 251 citizen scientists in the online classification of the images (project ‘WildLIVE! – Entdecke die wilden Tiere Boliviens’; Wildlive Project, 2022) during April 2020–25 June 2021. For the initial pass (Step 1: Species assignment) citizen scientists processed the entirety of the camera-trap image dataset (92,917 images) and assigned them as empty or as containing any of the target species. We (RM, MB and MJ) then reviewed and revised all jaguar classifications made by the citizen scientists (Step 2: Expert review) and further identified the remaining records to the individual level based on unique coat colour patterns (Step 3: Jaguar individualization; Plate 1). In some cases, individual identification was not possible because of insufficient image quality, and we excluded these records from the population structure and population abundance analyses. We assigned maturity as either adult (large, single individuals) or juvenile (small body size and/or if accompanied by an adult) and sex according to visible genitalia or repeated sightings of an adult individual with juvenile(s), which we took as evidence of a female. We assigned offspring to a specific female if we recorded them in the same capture event.
Camera-trap data analysis
From a total of 2,869 images labelled by citizen scientists as featuring jaguars we first discarded 127 (4.4%) with erroneous timestamps resulting from technical problems. To avoid multiple counting, we discarded images with repeated identification of the same individual or a record of an unidentified individual at the same station within 60 minutes and applied temporal autocorrelation (Silveira et al., Reference Silveira, Jácomo and Diniz-Filho2003; Foster et al., Reference Foster, Sarmento, Sollmann, Tôrres, Jácomo and Negrões2013). We used the remaining 437 capture events to fit an activity curve. To investigate variation in activity relative to sunrise/sunset, we used historical solar cycle data obtained from the suncalc package in R (Thieurmel & Elmarhraoui, Reference Thieurmel and Elmarhraoui2022). We grouped capture events according to time of day into three categories: day (8.00–18.00), night (20.00–5.00) and twilight (18.00–20.00 and 5.00–8.00; Jędrzejewski et al., Reference Jędrzejewski, Vivas, Abarca, Lampo, Morales and Gamarra2021).
We characterized population composition in terms of sex ratio, kinship and individual presence after discarding 93 images (3.4%) where unambiguous identification was impossible because of insufficient image quality or an unsuitable photo angle. We calculated the adult sex ratio as the ratio of adult males to adult females in the study area per year. We visualized individual-based capture events (including unidentified individuals) per station in terms of capture frequency relative to sampling effort (number of captures divided by the number of active trap-nights per station × 1,000; Botts et al., Reference Botts, Eppert, Wiegman, Rodriguez, Blankenship and Asselin2020).
We estimated jaguar density using maximum likelihood spatially explicit capture–recapture models using the secr package in R (Efford, Reference Efford2023). This approach estimates density over a defined space following a hierarchical multi-component model that includes a state model to describe the spatial distribution of the home range centre of an animal and an observation model to describe detections relative to the distance between the home range centre and the detector (Borchers & Efford, Reference Borchers and Efford2008). Such estimates are often calculated for short survey periods because closed capture–recapture models assume that no births, deaths or migration events occur during the sampling period (Kendall, Reference Kendall1999). However, extending survey periods from traditional values of, for example, 90 days to 180 days has been shown to improve precision and stability when estimating densities of long-lived mammals across multiple iterations (Dupont et al., Reference Dupont, Milleret, Gimenez and Bischof2019; Harmsen et al., Reference Harmsen, Foster and Quigley2020). We selected seven overlapping periods of 180 consecutive days in monthly intervals during 1 January–1 July 2019 (Harmsen et al., Reference Harmsen, Foster and Quigley2020). Two consecutive sessions had a mean overlap of 83.2%, which decreased by a mean of 17.0% for each session in-between. Every session included temporally independent captures of adult jaguars, as cubs of solitary felids generally have low capture probabilities (Karanth, Reference Karanth1995) and cannot be considered independent of their mother. We generated capture histories of every 180-day session whilst accounting for potential differences in sampling effort per station (e.g. if the cameras per station differed in their activity because of technical problems) and fitted spatially explicit capture–recapture models to every session. We fitted every model using a half-normal detection function and the default model structure. We defined the area of interest by a buffer size of at least five times the initial estimate of the root pooled spatial variance (Slade & Swihart, Reference Slade and Swihart1983), which was 6,589–9,643 m (mean 8,169 ± SD 1,487 m). We did not include sex as an individual-related covariate because distinguishing between sexes reduced the number of captures available to estimate density per session, resulting in a poor model fit.
We tested whether jaguar captures in Array B in 2019 differed significantly between land uses, using the Wilcoxon rank sum test (with α = 0.05). As open landscapes and some forest patches are used as pastures, we classified the type of land use at each camera station as being either pasture (⩾ 5 livestock capture events) or non-pasture (< 5 livestock capture events) for this analysis.
Vegetation cover analysis
We acquired three cloudless Sentinel-2 images of the study area from EarthExplorer (USGS, 2022) for 2017 (early study period), 2019 (late study period) and 2021 (after survey concluded and extensive deforestation had taken place). All 10-m resolution images were from 15–20 August, to avoid seasonal bias (Coppin et al., Reference Coppin, Jonckheere, Nackaerts, Muys and Lambin2004). We processed the images to show the normalized difference vegetation index within a range between −1 and 1 (Aburas et al., Reference Aburas, Abdullah, Ramli and Ash'aari2015) using QGIS 3.4.5 (QGIS, 2019). Negative values refer to a lack of vegetation cover, whereas positive values refer to different rates of vegetation density. We assigned the normalized difference vegetation index for the study area to five classes ranging from no vegetation to dense vegetation cover. We used the resulting cover density raster files to calculate the decrease in vegetation cover in the study area over a 5-year period (Aburas et al., Reference Aburas, Abdullah, Ramli and Ash'aari2015).
Results
Population structure, life history trajectories and activity
We analysed a total of 20,351 trap-nights and assigned individual identifications to all jaguar capture events if image quality was sufficient. Furthermore, we inferred reproduction, mortality and activity patterns based on individual occurrence data. We documented 437 independent capture events (12.85 ± SD 9.83 per month) throughout the study period (March 2017–December 2019, Array A plus Array B) and the detected number of jaguar individuals per month (= minimal estimate) varied between 1 and 5 (mean 2.76 ± SD 0.99; Fig. 2). Amongst 15 identified jaguars we recorded six males, four females and five juveniles. We inferred four reproduction events (five cubs) and three deaths (one confirmed death of an adult whose skin was found, and the likely death of two juveniles that disappeared before reaching independence; Fig. 2; Supplementary Plate 1). Jaguars in our study area followed a mostly nocturnal and crepuscular activity pattern, with 48% of records occurring at night and 40% at twilight (Fig. 3).

Fig. 2 Individual life histories as illustrated by capture histories of 15 jaguars over 3 years in the Chiquitano Dry Forest. Linked bands depict mother–offspring relationships. F, female; M, male; J, juvenile; the cross symbol indicates the confirmed death of an individual.
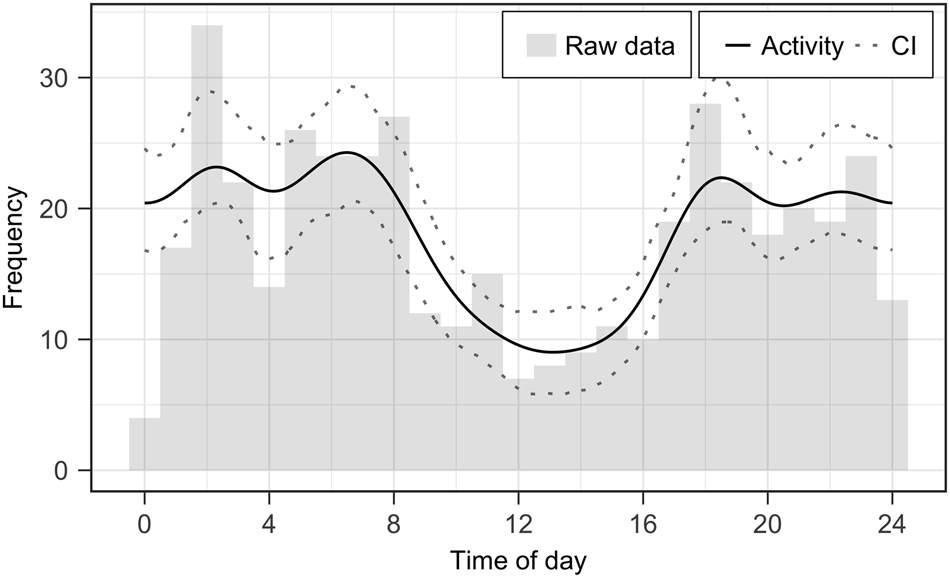
Fig. 3 Circadian activity patterns of jaguars in the Chiquitano region of the eastern Bolivian lowlands, including all pooled capture events from camera-trap Arrays A and B (Fig. 1).
Density and spatial distribution
Based on 437 independent jaguar capture events we estimated the population densities for both camera arrays using the same spatially explicit capture–recapture model and we inferred spatial distributions based on abundances relative to sampling efforts. Density estimates ranged from 1.32 ± SE 1.86 to 3.57 ± SE 2.58 jaguars per 100 km2 (Fig. 4; Supplementary Table 2). Jaguar detection frequency in relation to sampling effort and independent jaguar captures was highest in the south-western, forested section of the study area (Fig. 5). Land-use classification based on livestock observations indicate that jaguars occupy forest patches that are not used as pasture significantly more than areas often used for livestock grazing (p = 0.021, effect size r = 0.72; Supplementary Fig. 3). We recorded few to no jaguars in transformed agricultural land (Fig. 5).

Fig. 4 Jaguar density estimates, with confidence intervals, of all seven survey sessions (consecutive survey periods of 180 days in monthly intervals) in 2019 in the Chiquitano region of the eastern Bolivian lowlands.

Fig. 5 Temporally independent jaguar captures per station (pie charts) and integrated over the entire study period (bar chart) in the Chiquitano region of the eastern Bolivian lowlands. The pie chart sizes are scaled by jaguar abundance relative to sampling effort. White points (⊗) indicate stations with no captures. The diameters of the pie charts reflect sampling effort and the numbers of individuals per chart are not directly comparable between stations. We obtained the base map from Google (2022). F, female; M, male; J, juvenile. (Readers of the printed journal are referred to the online article for a colour version of this figure.)
Land-use change
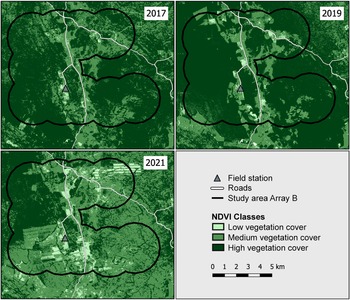
We created three normalized difference vegetation index maps of our study area to visualize the vegetation change (Fig. 6). We made two normalized difference vegetation index maps for the survey period (2017–2019) and for the recent condition of the area (2021). We observed major deforestation and conversion activities during 2017–2021 and the ratio of dense vegetation cover within the study area decreased by 33% between the two periods (Fig. 6). In contrast, moderately densely vegetated areas increased by 26% and areas with a low vegetation density increased by 7%. We estimate that c. 46.48 km2 of forest within the study area was cleared or degraded severely over this 5-year period.

Fig. 6 Normalized difference vegetation index (NDVI) for vegetation cover classes in August 2017, August 2019 and August 2021 in the Chiquitano region of the eastern Bolivian lowlands. Black contour shows Array B (active during March–December 2019) as the reference for calculating vegetation cover change.
Discussion
Our study area harbours a productive jaguar population. Of the 10 recorded adult jaguars, three females and two males appeared to be resident individuals as they were captured repeatedly by the camera traps. At least two of five cubs died before reaching adulthood and only two juveniles were recaptured independent from their mothers in later life stages.
The adult sex ratio (male : female) increased over the study period (first year: 1.0 : 2.0, second year: 1.0 : 1.5, third year: 1.0 : 0.4). More studies have identified male-biased (Maffei et al., Reference Maffei, Cuéllar and Noss2004; Soisalo & Cavalcanti, Reference Soisalo and Cavalcanti2006; Salom-Pérez et al., Reference Salom-Pérez, Carrillo, Sáenz and Mora2007), than female-biased (Moreno et al., Reference Moreno, Kays and Samudio2006; Tobler & Powell, Reference Tobler and Powell2013) jaguar sex ratios, which we observed only in the final year of our study. However, the observed shift towards more males in 2019 probably resulted from extending the surveyed area to achieve better representation of the sampled population rather than documenting an actual shift in the sex ratio over time. Males and females differ in their spatial use of habitats (Conde et al., Reference Conde, Colchero, Zarza, Christensen, Sexton and Manterola2010; Maffei et al., Reference Maffei, Noss, Silver, Kelly, O'Connell, Nichols and Karanth2011), which causes spatial heterogeneity of sex ratios within a population, with small survey areas not necessarily being representative of the sampled population. Although our initial restricted setup (Array A) covered a core habitat for resident females (as it contained a protected forest that is particularly suited for females to raise offspring, as evidenced by frequent mother–cub captures), the later expansion of the survey area into a larger, mixed landscape (more representative of the region; Array B) shifted the observed sex ratio towards males, which are more tolerant of human-modified landscapes (Conde et al., Reference Conde, Colchero, Zarza, Christensen, Sexton and Manterola2010). Because we set up Array A based upon anecdotal reports of frequent jaguar reproduction events, this female bias is unsurprising. In contrast, Array B used a systematic grid layout and covered a larger fraction of the sampled population. Yet even the 2019 sex ratio, similarly to other sex ratio estimates using similar methodology, is potentially biased by known sex-specific differences in jaguars. It is assumed that males are captured more frequently by camera traps as they have larger territories, are more mobile (Crawshaw & Quigley, Reference Crawshaw and Quigley1991; McBride & Thompson, Reference McBride and Thompson2018) and are more likely to disperse over greater distances (Kantek et al., Reference Kantek, Trinca, Tortato, Devlin, de Azevedo and Cavalcanti2021), causing a general bias towards capturing male jaguars.
Little is known about reproduction in wild jaguars, but our survey provides some insights into this. During March 2017–December 2019 we found evidence of four reproduction events involving three females. Three litters consisted of single cubs and one consisted of two cubs. Previous geographically diverse jaguar studies have documented similar sized litters and attributed them to low conception rates or high infant mortality (Carrillo et al., Reference Carrillo, Saenz and Fuller2009; Cavalcanti & Gese, Reference Cavalcanti and Gese2009; Cuéllar et al., Reference Cuéllar, Alarcón, Peña, Méndez, Romero-Muñoz and Maffei2012). One female reproduced twice, with c. 16 months between the first records of the first and second litters. The results from a previous radiotelemetry-based study estimated a 22–24 month breeding interval for wild jaguars (Carrillo et al., Reference Carrillo, Saenz and Fuller2009).
Our data indicate that there is no mating season for jaguars, which is consistent with previous studies (Cavalcanti & Gese, Reference Cavalcanti and Gese2009; Beisiegel et al., Reference Beisiegel, Sana and Moraes2012; Cavalcanti et al., Reference Cavalcanti, Azevedo, Tomás, Boulhosa and Crawshaw2012; Harmsen et al., Reference Harmsen, Foster and Quigley2020). The occurrence of females and cubs was concentrated in forested areas, suggesting that these patches provide better conditions for raising offspring than a more disturbed and fragmented environment. This is in line with previous studies and has been associated with higher prey abundances compared to open landscapes (Weckel et al., Reference Weckel, Giuliano and Silver2006; Conde et al., Reference Conde, Colchero, Zarza, Christensen, Sexton and Manterola2010). We found no evidence for seasonal spatial avoidance between females as suggested previously (Cavalcanti & Gese, Reference Cavalcanti and Gese2009), but some evidence for avoidance between males: during the first survey year an increased capture frequency of a known resident male (M-02) corresponded with fewer sightings of a second known male (M-01), and the disappearance of the first male (July 2018) resulted in higher capture frequencies of the second male.
Jaguars exhibited primarily nocturnal and crepuscular activity periods, with peaks in activity around dusk/dawn and at 2.00/3.00. Although several previous studies found similar activity patterns in Bolivia (Maffei et al., Reference Maffei, Noss, Silver, Kelly, O'Connell, Nichols and Karanth2011), others report deviating patterns throughout the distribution range of the jaguar, supporting the notion of there being flexibility in the circadian activity of the species (Botts et al., Reference Botts, Eppert, Wiegman, Rodriguez, Blankenship and Asselin2020). This variation in jaguar activity patterns could be correlated with habitats, prey and competition (Harmsen et al., Reference Harmsen, Foster and Doncaster2011; Botts et al., Reference Botts, Eppert, Wiegman, Rodriguez, Blankenship and Asselin2020). Additionally, sex and reproduction could affect jaguar activity further, with reproductive females showing higher daytime activity levels than adult males, non-reproductive females and cubs (Jędrzejewski et al., Reference Jędrzejewski, Vivas, Abarca, Lampo, Morales and Gamarra2021).
Our estimated jaguar densities support the findings of previous studies in the Chiquitano Dry Forest (Supplementary Table 3), and provide further support that unprotected areas with intact natural habitats, especially cattle ranches, are crucial habitats for jaguars (Rumiz et al., Reference Rumiz, Arispe, Noss and Rivero2003; Arispe et al., Reference Arispe, Rumiz and Venegas2005; Maffei et al., Reference Maffei, Noss, Silver, Kelly, O'Connell, Nichols and Karanth2011; Jędrzejewski et al., Reference Jędrzejewski, Robinson, Abarca, Zeller, Velasquez and Paemelaere2018). Ecological research on jaguars to date has focused on protected areas and only 27 of 131 of available density estimates were obtained from unprotected areas (Foster et al., Reference Foster, Harmsen, Urbina, Wooldridge, Doncaster and Quigley2020). Density estimates using spatially explicit capture–recapture in small study areas over short time frames require cautious interpretation. However, the reliability of density estimates can be strengthened by providing estimates acquired over multiple survey sessions throughout a year (Harmsen et al., Reference Harmsen, Foster and Quigley2020). We provide a range of density estimates using a 180-day sample period taken over 12 months instead of the often used single 50–70 day survey period (Jędrzejewski et al., Reference Jędrzejewski, Vivas, Abarca, Lampo, Morales and Gamarra2021). To date, population closure in jaguars is understood poorly and by extending the survey period to 180 days the value of the data gained is believed to outweigh the risk of closure violation (Tobler & Powell, Reference Tobler and Powell2013).
We did not record jaguars in deforested agricultural land, indicating that they avoid such areas. However, jaguars are able to persist within agricultural regions that retain intact forested areas and where hunting of both jaguars and their prey is limited (Boron et al., Reference Boron, Tzanopoulos, Gallo, Barragan, Jaimes-Rodriguez and Schaller2016). Jaguar activity depends on forest coverage and prey availability, the latter of which is likely to be linked to the opportunistic foraging behaviour of jaguars (Weckel et al., Reference Weckel, Giuliano and Silver2006; McBride & Thompson, Reference McBride and Thompson2018).
Deforestation and the number of large wildfires in the Brazilian Amazon have significant consequences for biodiversity and displace hundreds of jaguars each year (Menezes et al., Reference Menezes, Tortato, Oliveira-Santos, Roque and Morato2021). Widespread wildfires in 2019 burned 20,000 km2 of the Chiquitano Dry Forest, killing an estimated 5.9 million mammals (Pacheco et al., Reference Pacheco, Quispe-Calle, Suárez-Guzmán, Ocampo and Claure-Herrera2021). These fires affected our study area in August 2019 and were followed by the second-lowest estimate of jaguar density.
Protected areas serve as important refuges for large carnivores but may not be large enough to sustain viable populations (Rabinowitz & Zeller, Reference Rabinowitz and Zeller2010; Boron et al., Reference Boron, Tzanopoulos, Gallo, Barragan, Jaimes-Rodriguez and Schaller2016). The jaguar in particular is at risk of displacement, population decline and local extinction (Menezes et al., Reference Menezes, Tortato, Oliveira-Santos, Roque and Morato2021). Previous studies have documented healthy and rich ecosystems in the Chiquitano Dry Forest (Rumiz et al., Reference Rumiz, Fuentes, Rivero, Santiváñez, Cuéllar and Miserendino2002; Arispe et al., Reference Arispe, Rumiz and Noss2007; Venegas et al., Reference Venegas, Rumiz, Angulo and Rivero2010; Polisar et al., Reference Polisar, de Thoisy, Rumiz, Díaz Santos, McNab and Garcia-Anleu2016; Jansen et al., Reference Jansen, Engler, Blumer, Rumiz, Aramayo and Krone2020). Our results further support the notion that the Chiquitano Dry Forest in Bolivia, which is often embedded in a mixed land-use area, harbours a significant but still understudied population of jaguars.
Our investigation reports a productive jaguar site on privately owned lands just before the destruction of much of this habitat through deforestation. Of the 263,000 km2 of potential jaguar habitats in the Department of Santa Cruz, 55% occurs on privately owned properties that harbour potentially productive jaguar sites (Maillard et al., Reference Maillard, Angulo, Vides-Almonacid, Rumiz, Vogt and Monroy-Vilchis2020). Future regional landscape management should focus on the connectivity between conservation units, to preserve wildlife in fragmented areas (Hess & Fischer, Reference Hess and Fischer2001; Petracca et al., Reference Petracca, Hernández-Potosme, Obando-Sampson, Salom-Pérez, Quigley and Robinson2014). In the Chiquitano region, where c. 5,700 cattle properties include potential jaguar habitat (Maillard et al., Reference Maillard, Angulo, Vides-Almonacid, Rumiz, Vogt and Monroy-Vilchis2020), private landowners are in a position to make significant contributions to the protection of the Chiquitano Dry Forest. Regional efforts to counteract the ongoing biodiversity crisis should therefore involve local stakeholders in participatory and co-creational processes aiming to increase responsible and sustainable land-use management. This could include incentives for green labelling and ecotourism (Amit & Jacobson, Reference Amit and Jacobson2018; Hyde et al., Reference Hyde, Boron, Rincon, Passos Viana, Larcher, Reginato and Payán2022) and strengthen the socio-cultural identity of Indigenous communities regarding nature. Comprehensive monitoring of the immediate effects of deforestation on the population dynamics of indicator species in mixed land-use areas will be essential to inform and guide future actions to conserve the remaining intact ecosystems of the eastern Bolivian lowlands, and the Neotropics in general.
Acknowledgements
We thank the cattle ranch owners (Domingo Nazer, Roger Alejandro Parada Ortiz, Ángel Belaunde, María E. Suarez de la Belaunde, Eduardo Jordán and Miguel Antelo) for their cooperation, and our collaborators in the field (Israel Costaleito, Leidy Chamu, Mario Cerón Altamirano, Leonor Guayacuma, Wilber Daza Humaza, Corcino Ramos, Eugenio Mallco, Eunice Copa, Emanuel Crespo, Sissy Suarez and Martha Córdova). Analysis of camera-trap data was made possible through the engagement of many citizen scientists (Supplementary Table 4). We thank the citizen scientists Anja Czoelder, Jan Göpel, Alexandra König, Carmen Spalke, Alexandra Ottong and Peter Zehner for help with data transfer, and the editor and two anonymous reviewers for their constructive critiques. Alfredo Romero Muñoz provided useful comments on a previous version of this text and Oswaldo Maillard contributed additional information. This study was funded by the Deutsche Forschungsgemeinschaft 437771903, and by BIOPAT e.V., Darmstadt, Germany. The study was performed under the agreement between the Museo Historia Natural Noel Kempff Mercado, Santa Cruz, and the Senckenberg Research Institute and Nature Museum, and approved by the Directorate of Biodiversity, Ministry of Environment and Water, Plurinational State of Bolivia.
Author contributions
Study management and supervision: MJ; study design: RM, MBI, MJ; raw data management: JLAB; data collection: YCC, JLAB, GAL, RM, MBI, MJ; individual jaguar identification: RM, MBI, MJ; data analysis: MW, MBe, MBI, MJ, RM; writing: MJ, RM, MBI, MBe, MW; figures: MW, MBe, MBI, RM, MJ, GAL; external funding: MJ.
Conflicts of interest
None.
Ethical standards
This research abided by the Oryx guidelines on ethical standards. Our handling and use of camera-trap data abided by the recommended code of conduct for such research (Sharma et al., Reference Sharma, Fiechter, George, Young, Alexander and Bijoor2020).