Introduction
The guanaco Lama guanicoe is the dominant wild herbivore in the Andean steppe and plays a central role in South American arid ecosystems. It is a social species, with a social structure that includes family groups, bachelor groups and single males. Franklin & Fritz (Reference Franklin, Fritz, Robinson and Redford1991) described sedentary and migratory guanaco populations, indicating that populations tend to be sedentary where forage resources are easily defensible, allowing territorial males to maintain their territories throughout the year. In contrast, where forage and water are limited and weather conditions are adverse in some seasons, groups or individuals do not have permanent territories but migrate between seasonal ranges.
Guanaco populations have declined by > 90% throughout most of their range during the last 100 years (Baldi et al., Reference Baldi, Novaro, Funes, Walker, Ferrando, Failla, Carmanchahi, du Toit, Kock and Deutsch2010). Habitat desertification as a result of overgrazing by domestic livestock has occurred in the arid Patagonia region of Argentina, which holds the greatest abundance of guanacos. Other factors in the species' decline include forage competition with sheep, indiscriminate legal and illegal hunting, and the negative perception of the guanaco among local people (Raedeke, Reference Raedeke1979; Baldi et al., Reference Baldi, Albon and Elston2001, Reference Baldi, Novaro, Funes, Walker, Ferrando, Failla, Carmanchahi, du Toit, Kock and Deutsch2010). With the inclusion of the guanaco in Appendix II of CITES in 1993 (CITES, 2013) it was recommended that countries did not import guanaco products from Argentina, which resulted in a decline in commercial hunting of guanacos. Argentinian legislation only allows the export of guanaco fibre obtained from live individuals, which can be achieved by breeding animals in captivity or by management of wild guanacos. Private and government initiatives have been developed to obtain fibre through capture, shearing and subsequent release of wild guanacos (Montes et al., Reference Montes, Carmanchahi, Rey and Funes2006; Carmanchahi et al., Reference Carmanchahi, Ovejero, Marull, López, Schroeder and Jahn2011). However, stress associated with these activities in such a social species may reduce its reproductive success and resilience, and therefore knowledge of the biological effects of live-shearing, both on migratory and sedentary populations, is required.
Studies of the physiological effects of shearing have shown that stress levels, measured through cortisol levels, increase significantly with handling time during capture and shearing of wild guanacos (Carmanchahi et al., Reference Carmanchahi, Ovejero, Marull, López, Schroeder and Jahn2011). In addition, behavioural indicators show an increase in stress levels among larger groups of guanacos in round-up corrals (Taraborelli et al., Reference Taraborelli, Ovejero, Schroeder, Moreno, Gregorio and Carmanchahi2011). However, mortality during shearing is generally low (Baldi et al., Reference Baldi, Novaro, Funes, Walker, Ferrando, Failla, Carmanchahi, du Toit, Kock and Deutsch2010; Rey et al., Reference Rey, Novaro, Sahores and Guichón2012b). There is limited information available about the effects of management on population parameters and behavioural responses after guanacos are released, and no data on effects on migratory populations.
Our objective is to evaluate the effects of round-up and shearing on density, group size, spatial distribution, survival, reproduction and movement in sedentary and migratory populations of wild guanacos in northern Patagonia, Argentina. We hypothesize that shearing of wild guanacos affects these parameters less in populations living in open areas with few barriers than in sedentary populations in areas with fences for livestock, which impose restrictions on daily and seasonal movements in search of forage.
Study area
We conducted our study at two sites where capture and handling of wild guanacos have occurred since 2005, following the methodology described in Carmanchahi et al. (Reference Carmanchahi, Ovejero, Marull, López, Schroeder and Jahn2011) and Taraborelli et al. (Reference Taraborelli, Ovejero, Schroeder, Moreno, Gregorio and Carmanchahi2011). Management conditions at the sites differ in terms of the number of guanacos shorn per year, livestock densities, presence of wire fences, and land ownership.
The first site is a sheep ranch in Río Negro province (Fig. 1), which harbours a sedentary population of wild guanacos (20.4 ± SE 2.7 guanacos per km2; P.D. Carmanchahi & M. Funes, unpubl. data). The ranch covers 400 km2 (divided by a 1.3 m high fence into seven paddocks of 50 km2 and two of 25 km2), with c. 25 sheep per km2 (P.D. Carmanchahi & M. Funes, unpubl. data). Guanacos are captured and shorn in four of the paddocks, where capture and shearing structures are in place year-round. Vegetation is characterized by low shrubby steppe, with a transition between Patagonian Steppe and Monte Desert phytogeographical provinces (León et al., Reference León, Bran, Collantes, Paruelo and Soriano1998). Small canyons, tablelands and valleys dominate the physiography. This region has a desert climate, with mean annual precipitation of < 200 mm, concentrated in autumn and winter. Mean annual temperature is 10–12°C.

Fig. 1 The study sites in Argentina where we investigated the effects of capture and shearing on populations of guanacos Lama guanicoe: a sheep ranch in Río Negro province (1), and La Payunia Provincial Reserve, in Mendoza province (2).
The second site is La Payunia Provincial Reserve, in southern Mendoza province (Fig. 1). This region is a semi-arid biome of the La Payunia phytogeographical province within the Andean–Patagonian domain (Martínez Carretero, Reference Martínez Carretero2004) and has a desert climate, with mean annual precipitation of 255 mm and a mean temperature of 6°C in winter and 20°C in summer. The Reserve covers 6,400 km2, of which 2,000 are state-owned lands, and supports the largest known migratory population of guanacos in central Argentina, with up to 26,000 individuals in spring (Schroeder, Reference Schroeder2013). Mean annual livestock densities for the northern part of the Reserve (c. 1,200 km2) were estimated at 7.89 km−2 for small livestock (goats and sheep) and 3.55 km−2 for large livestock (cattle and horses; Schroeder, Reference Schroeder2013).
Every year, the provincial wildlife agency authorizes a cooperative of small-scale herders who inhabit the periphery of the Reserve to capture and shear guanacos. As this occurs inside a protected area a mobile structure for capture and handling of guanacos and a temporary camp must be set up by the herders and dismantled completely afterwards. The camp houses c. 40 herders for 25–30 days.
Methods
Wild guanacos were rounded up and driven into a corral trap (described in Carmanchahi et al., Reference Carmanchahi, Ovejero, Marull, López, Schroeder and Jahn2011) by horse riders, and handled according to a welfare protocol (Carmanchahi & Marull, Reference Carmanchahi and Marull2012) approved by the Wildlife Departments of Mendoza and Río Negro provinces and the National Wildlife Department. Shorn individuals were fitted with coloured, numbered collars and then released.
We conducted ground surveys of guanacos at both study sites, using the line-transect method (Buckland et al., Reference Buckland, Anderson, Burnham, Laake, Borchers and Thomas2001), making observations from the back of an open truck while driving along secondary roads and trails at 10–30 km per hour. We recorded the number of guanacos in each group, the radial distance to the animal or to the centre of the group (measured with a laser range-finder with accuracy ± 1 m), and the angle between the transect and the centre of the group (measured with a compass). We recorded the geographical coordinates of each sighting, using a global positioning system (GPS). At the sheep ranch the surveyed area was 250 km2, with a sampling effort of 81.7 km, and at the Reserve the surveyed area was 200 km2, with a sampling effort of 75 km.
At the sheep ranch capture and shearing were carried out at the post-partum stage (January) in 2005 and 2007. At the Reserve capture and shearing were carried out during the pre-partum stage (September–October) during 2005–2010 (except 2008). As guanaco offspring are already mobile and able to follow their mother in January we consider it valid to compare movement parameters and social organization of guanacos among populations shorn before and after the birthing period.
At the sheep ranch a search for dead guanacos was conducted on horseback 10 days after capture and shearing, and additional surveys were conducted by vehicle 15–30 days thereafter on all the trails within each paddock. Ground surveys and radio-collars were used to assess the survival of shorn guanacos at the Reserve. Ten adult males and 22 adult females were fitted with radio-collars (six GPS and 26 VHF) during 4 years (2005, 2006, 2007 and 2009). Guanacos were tracked on the ground by MJB and JB, daily during the first 2 weeks after capture and 2–3 times per week afterwards, and occasionally from a fixed-wing aircraft. We estimated the date of death and cause of mortality of radio-collared guanacos through visual inspection of carcasses, and assessed nutritional condition by examining the colour and consistency of femoral marrow fat (Harder & Kirkpatrick, Reference Harder, Kirkpatrick and Bookhout1994). We estimated the survival rate of radio-collared guanacos during the first year after round-up, using the Kaplan–Meier method (Pollock et al., Reference Pollock, Winterstein, Bunck and Curtis1989), combining data from round-up events during 2005–2009 to increase sample sizes.
We attempted to observe radio-collared guanacos during the breeding season, during 2005–2009, to determine the proportion of females with young after the animals were rounded up and shorn. However, it was difficult to approach radio-collared guanacos because of the paucity of trails in the Reserve. Less than 1% of triangulations led to sightings and we were able to assess the proportion of females with young for a sample of only 14 radio-collared females.
We assessed changes in activity by measuring daily distances moved and home range sizes of guanacos that were shorn and released in the Reserve. Given the low precision of VHF collars, daily distances moved are reported only for guanacos with GPS collars and home range sizes are estimated separately for guanacos with GPS and VHF collars. As we did not have movement data prior to capture and shearing or for non-shorn guanacos, we compared daily distances moved during the first 2 days after shearing and release to distances moved in the following 30 days, and compared home range sizes during the first 30 days after shearing with sizes 30–60 days after shearing. To assess whether changes in movement were associated with seasonal variation we also reported daily movements and home range sizes during the same periods the following year. We could not estimate monthly home ranges based on VHF data, as we did for GPS data, and therefore we grouped VHF data for the 2 months after shearing and compared this home range estimate with that for the same period the following year. Distances moved and home range sizes were compared using generalized linear models, considering guanacos as a random effect because of the high variability among individuals (Hoffmann, Reference Hoffmann2004).
To evaluate changes in the parameters of the migratory population we conducted surveys at the Reserve, considering the camp installation and setting up of the capture structure as disturbance factors. We carried out pre-camp surveys 3 days before starting to build the capture structure and the camp, and pre-capture surveys 2 days before the round-up and capture events. We performed surveys 1–2 days after capture, shearing and release, to detect immediate effects, and c. 1 month later to detect long-term effects. At the sheep ranch we only tested long-term effects, conducting post-shearing surveys 15–30 days after shearing.
We used Distance v. 5.0 (Buckland et al., Reference Buckland, Anderson, Burnham, Laake, Borchers and Thomas2001) to calculate individual and group density (guanacos per km2 and groups per km2, respectively) and group size (guanacos per group). We chose the best model based on the Akaike information criterion, goodness-of-fit tests and visual assessment of data in histograms (Buckland et al., Reference Buckland, Anderson, Burnham, Laake, Borchers and Thomas2001). We investigated whether detection probability was related to group size, as this can result in overestimation of density. We carried out a regression among the natural logarithms of group size and the detection probability of each group, with a significance level of P < 0.15 (Buckland et al., Reference Buckland, Anderson, Burnham, Laake, Borchers and Thomas2001). We used ANOVA to compare group and guanaco densities and mean group size pre- and post-shearing at the sheep ranch, and pre-camp, pre-capture, and immediate and late post-shearing at the Reserve. Post-hoc testing (Tukey test, P < 0.05) was used to test for differences among variables.
To assess whether the installation of the camp and capture events influenced the spatial distribution of guanacos we divided the study area into regular intervals of 1 km (sheep ranch) and 2 km (Reserve) from the shearing corral. Different spatial resolution was used at the two sites because of differences in the number of individuals observed and their natural dispersion. We recorded the number of individuals and the mean group size along transects within each distance interval. We standardized the observations according to the number of kilometres surveyed per distance interval. For the sheep ranch we evaluated the effect of three independent capture events in three paddocks during 2005. As wire fences are semi-permeable barriers that cause direct mortality and are rarely crossed by wild guanacos (Rey et al., Reference Rey, Novaro and Guichón2012a), we considered the guanaco subpopulations of each paddock to be independent and analysed each as a replicate. For the Reserve we evaluated the effect of camp installation during 2009 and 2010. For each year we used portions of ground surveys of 0.7–7.4 km (2009) and 1.3–10 km (2010) as sampling units. To ensure independence, fragments were separated by at least 1 km. We used a Friedman non-parametric block ANOVA to compare the number of individuals and group size per km pre- and post-shearing at both sites. IDRISI Taiga v. 16.05 was used for spatial analyses and InfoStat (Di Rienzo et al., Reference Di Rienzo, Casanoves, Balzarini, Gonzalez, Tablada and Robledo2011) and R (R Development Core Team, 2012) for statistical analyses.
Results
Survival and reproduction
Of the 32 radio-collared guanacos in La Payunia Provincial Reserve, one female died 10 days after shearing in 2007, within the period of high risk of capture myopathy. The results of a necropsy indicated that the guanaco was in good physical condition, with no signs of disease or predation, but no signs of capture myopathy were detected in an analysis of tissue samples (V. Rago, pers. comm.). The remaining 31 guanacos survived during the year after capture. We therefore estimate an annual post-shearing survival rate of 0.98 ± SE 0.02.
At the end of the reproductive season (March–April) in the Reserve we were able to observe 9 out of 12 and 5 out of 6 adult females shorn and radio-collared in 2006 and 2007, respectively. Of these, two females radio-collared in 2006 and four radio-collared in 2007 had young, indicating an overall mean proportion of 0.43 during the study period.
Of the 658 guanacos captured in the Reserve during 2006, 2007, 2009 and 2010 only two shorn individuals were found dead during post-shearing surveys, and therefore we estimate a maximum post-shearing survival rate of 0.997 ± SE 0.001.
At the sheep ranch 2,934 guanacos were captured during five capture and shearing events, and five were subsequently found dead, yielding an estimated maximum post-shearing survival rate of 0.99 ± SE 0.01.
Movement
At the Reserve the mean daily distance moved by the six guanacos with GPS collars was 9.1 ± SE 1.94 km during the first 2 days after release and 3.6 ± SE 0.54 km during the following 30 days (F = 6.31, P = 0.05; random effect not significant: F = 0.848, P = 0.57). Four guanacos moved > 10 km (up to 17 km) during the first 2 days after capture. The mean daily distance moved by the six guanacos with GPS collars during the same month the following year was 2.9 ± SE 0.42 km.
The mean home range of the six guanacos with GPS collars was 220 ± SE 51 km2 during the month immediately after shearing and declined by 25%, to 166 ± SE 26 km2, during the following month, although this decline was not significant (F = 0.67, P = 0.45; random effect: F = 0.48, P = 0.78). The mean home range of the same guanacos during the month beginning 1 year after the shearing date was 187 ± SE 63 km2 (15% smaller than the previous year) and had declined by 31% a month later, to 129 ± SE 59 km2. Similarly, the mean home range of 25 guanacos with VHF collars declined by 23% between the 2 months immediately after shearing (169 ± SE 136 km2) and the same 2 months 1 year later (130 ± SE 132 km2; F = 2.178, P = 0.154), with significant differences among individuals (random effect: F = 3.728, P = 0.002; ranges were 33–596 km2 during the year of capture and 40–425 km2 during the following year). Females contributed more than males to this overall decline: 75% of females had second-year home ranges that were 35–74% smaller than in the first year, whereas only 30% of males had home ranges that were > 20% smaller than in the first year. For all other guanacos of both sexes home ranges during the second year were larger or < 20% smaller than in the first year.
Density and population structure
In the sedentary population at the sheep ranch there were no significant differences in group density (F = 0.14, P = 0.72, df = 1, n = 12; Fig. 2a), guanaco density (F = 1.79, P = 0.21, df = 1, n = 12; Fig. 2b) or mean group size (F = 1.75, P = 0.22, df = 1, n = 12; Fig. 2c) before and after round-up and shearing.
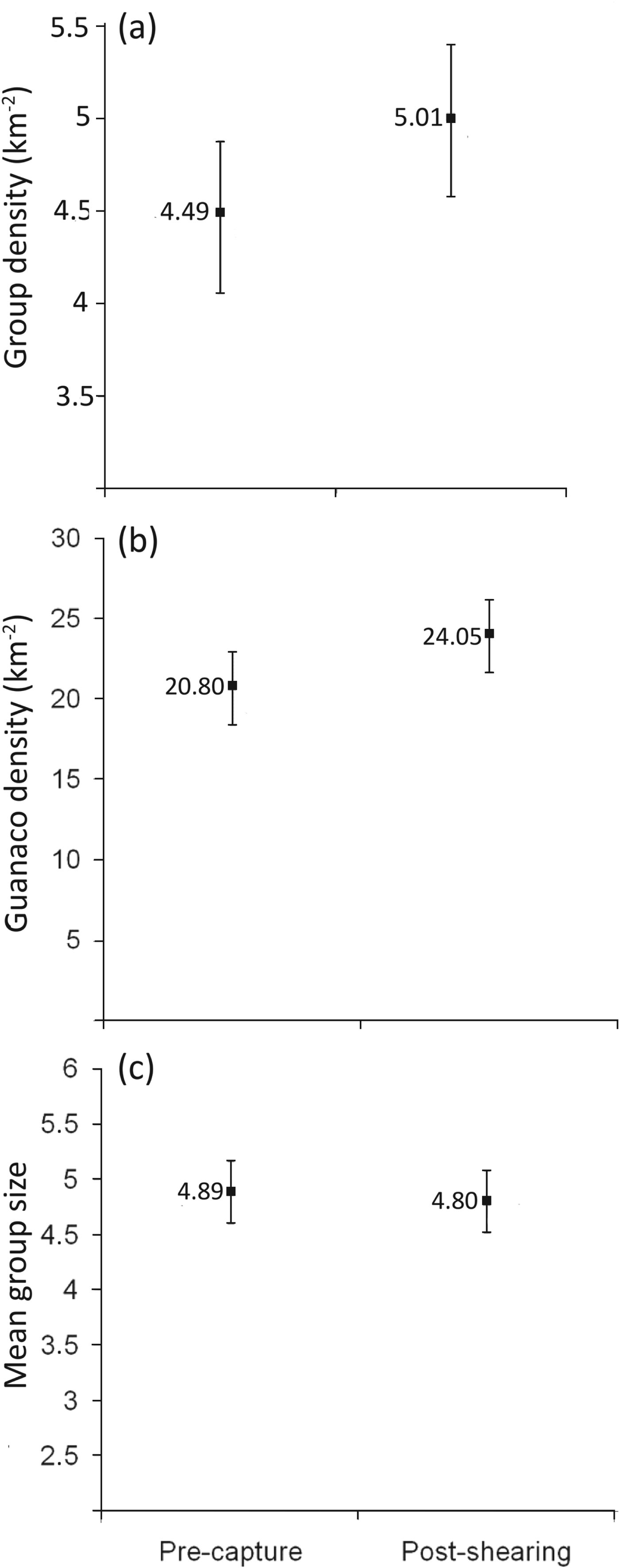
Fig. 2 Population parameters pre-capture and post-shearing for guanacos on the sheep ranch in Rio Negro province (Fig. 1): (a) group density, (b) population density, and (c) mean group size. None of the values are significantly different (post-hoc Tukey test, P < 0.05).
At the Reserve there was a significant decline in guanaco density (F = 19.46, P = 0.0001, df = 11, n = 4; Fig. 3b) and mean group size (F = 15.5, P = 0.0007, df = 9, n = 4; Fig. 3c) after installation of the camp and the capture structure, whereas a similar group density was maintained. Group density (but not guanaco density or group size) was significantly greater in surveys immediately post shearing (F = 19.95, P = 0.0003, df = 9, n = 4; Fig. 3a) compared to levels after installation of the camp and capture structure. One month after shearing, guanaco density was similar to that estimated prior to management activities (F = 19.5, P = 0.0001, df = 11, n = 4, Fig. 3b).

Fig. 3 Population parameters pre-capture and post-shearing for guanacos in La Payunia Provincial Reserve, Mendoza province (Fig. 1): (a) group density, (b) population density, and (c) mean group size. Different letters indicate P < 0.05 by post-hoc Tukey test.
Spatial distribution
At the sheep ranch pre-capture densities were greater than post-shearing densities up to 6 km from the shearing corral but these differences were significant only at 3 km (pre-capture: 35.86 ± SE 9.6 individuals per km; post-shearing: 12.24 ± SE 2.29 individuals per km; T 2 = 1E30, P < 0.0001; Fig. 4a) and at 6 km (pre-capture: 17.32 ± SE 3.05 individuals per km; post-shearing: 5.37 ± SE 0.61 individuals per km; T 2 = 1E30, P < 0.0001; Fig. 4a). The mean group size was significantly higher pre-capture (3.01 ± SE 0.56 individuals per group) than post-shearing (1.38 ± SE 0.24 individuals per group; T 2 = 1E30, P < 0.0001; Fig. 4b) only at 6 km from the shearing corral.
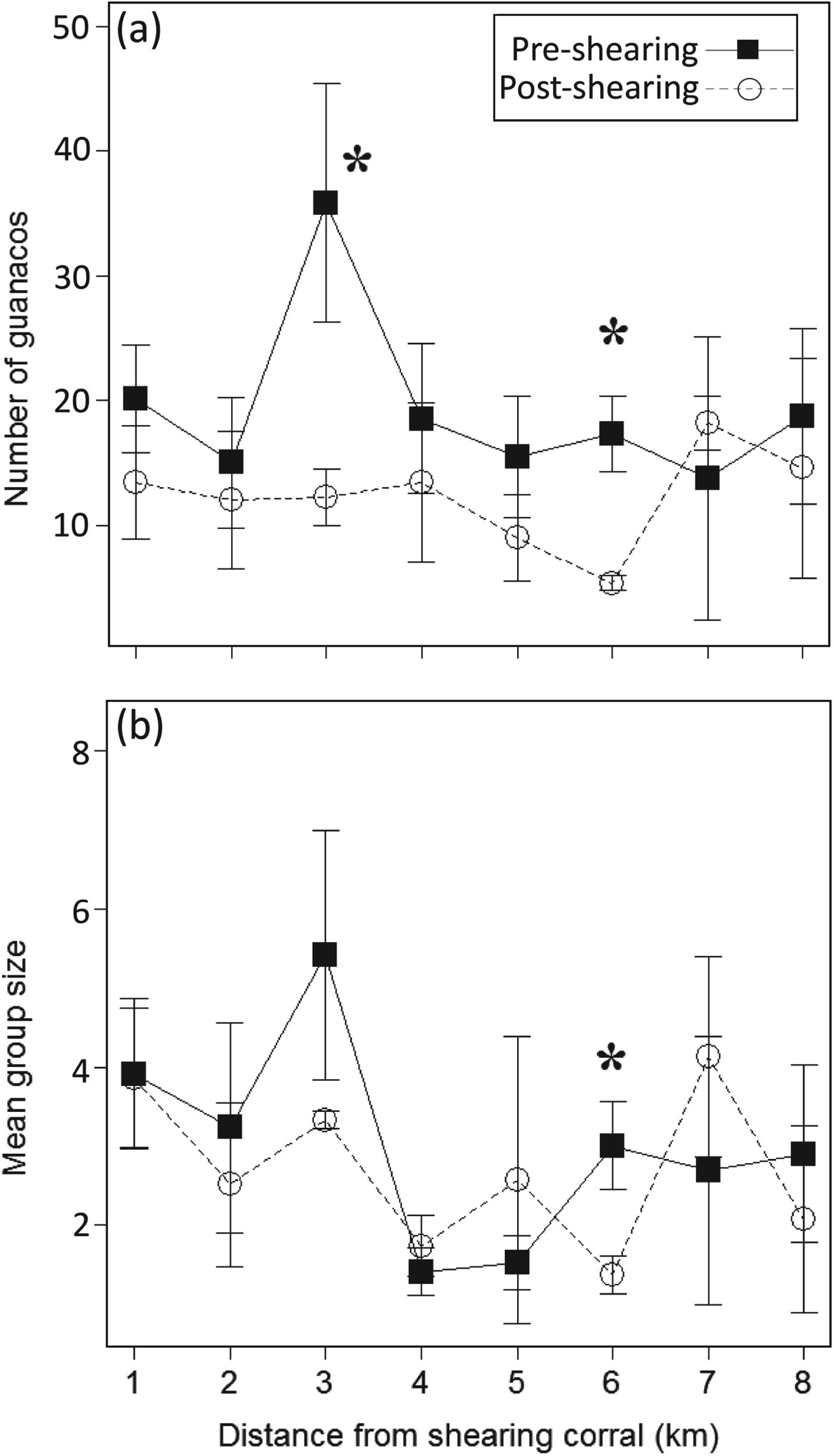
Fig. 4 Mean number of guanacos ± SE (a) and mean group size ± SE (b) with distance from the shearing corral before and after the shearing events on the sheep ranch during 2005.
At the Reserve pre-capture density was significantly lower before than after installation of the camp (0.78 ± SE 0.8 individuals per km and 10.36 ± SE 4.46 individuals per km, respectively; T 2 = 1E30, P < 0.0001; Fig. 5a) at 8 km from the shearing corral. Mean group size was also smaller before than after installation of the camp (0.79 ± SE ±0.8 individuals per group and 9.79 ± SE 4.8 individuals per group, respectively; T 2 = 1E30, P < 0.0001; Fig. 5b), only at 8 km from the shearing corral.
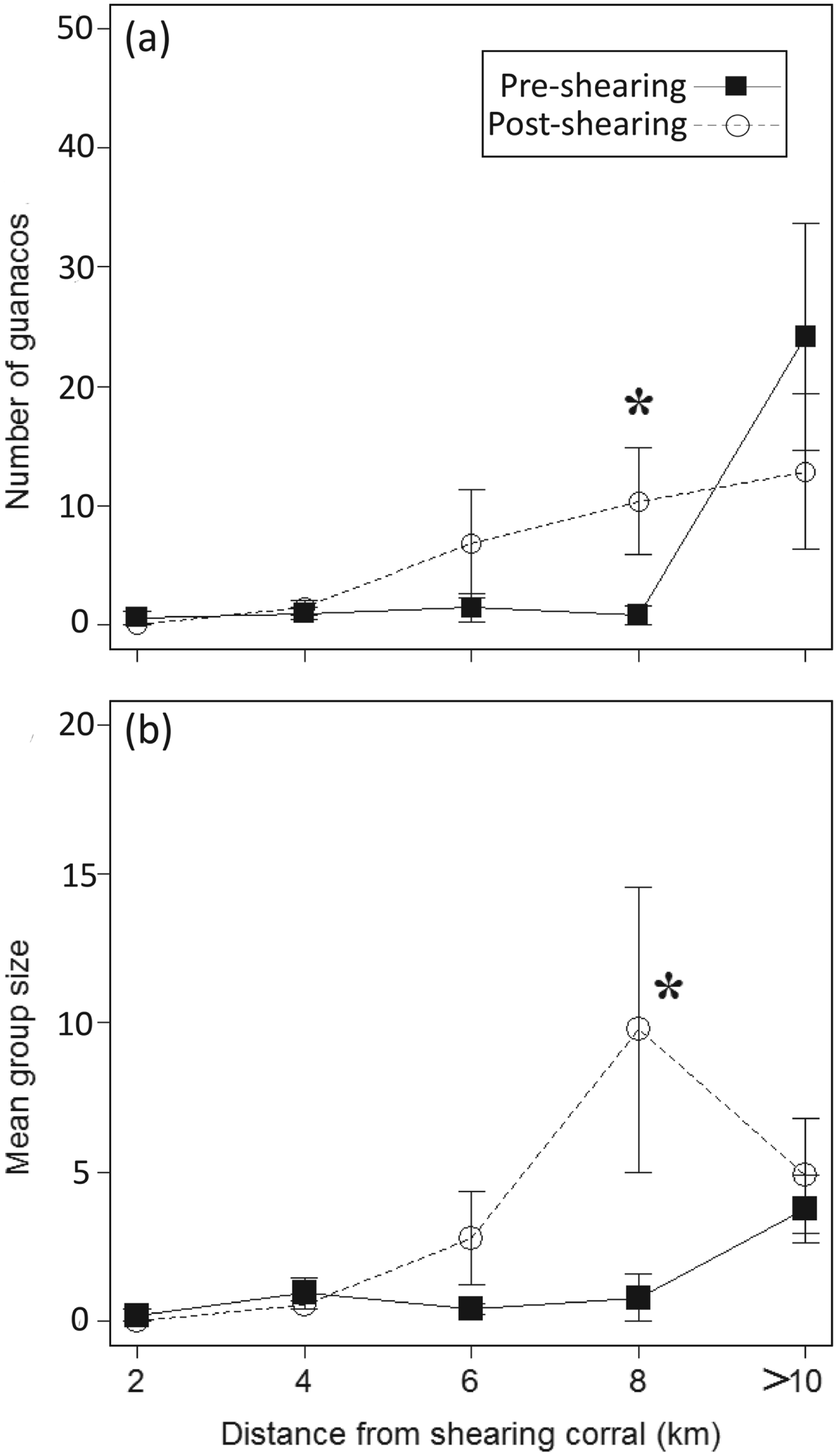
Fig. 5 Mean number of guanacos ± SE (a) and mean group size ± SE (b) with distance from the shearing corral before and after installation of the camp in La Payunia Provincial Reserve in 2009 and 2010.
Discussion
Effects on survival and reproduction
The survival rate of shorn guanacos in the Reserve was higher than the rates estimated in the only other reported study on shorn guanacos, at Cabeza de Vaca ranch (Rey et al., Reference Rey, Novaro, Sahores and Guichón2012b). At Cabeza de Vaca the annual survival rate estimated from telemetry of 17 shorn adult guanacos was 0.70 ± SE 0.11, whereas survival estimated from 1,334 capture–recapture histories was 0.82 ± SE 0.01. Lower survival at Cabeza de Vaca ranch than in La Payunia Reserve may be attributable to various factors, including restriction of guanaco movements by fences, competition with sheep (Rey et al., Reference Rey, Novaro and Guichón2012a) and differences in handling protocols.
Capture and shearing do not appear to have affected reproduction of guanacos in La Payunia, based on our results for radio-collared individuals. The mean proportion of yearlings associated with shorn radio-collared females at the end of the breeding season (0.43) was similar to the proportion of yearlings in the population, estimated from transect surveys during the same period (0.43 ± SE 0.21; M. Bolgeri & A. Novaro, unpubl. data). This similarity indicates that capture and shearing did not reduce the breeding success of radio-collared guanacos, or the early survival of their young.
Effects on movement, density and population structure
Capture stress may trigger a number of behavioural responses. These include flight-or-fight responses or development of behavioural strategies such as seeking refuge and waiting before returning, which was observed in roe deer Capreolus capreolus in response to capture, handling and fitting of a collar (Morellet et al., Reference Morellet, Verheyden, Angibault, Cargnelutti, Lourtet and Hewison2009). In contrast, our results indicate that live capture, shearing and release in the Reserve triggered dispersion by guanacos. After shearing there were smaller groups, indicating that some animals moved away from the disturbed area. Telemetry data indicated that there was increased movement during the days following capture, which may account for the larger home range sizes observed during the month after capture and shearing. This effect had not been described previously for wild populations of South American camelids and may have been initiated by the disturbance caused by setting up the camp and capture structures, and exacerbated by capture and shearing activities.
Increased movement in response to disturbance may only occur in some individuals, particularly in adult females, as evidenced by the larger reduction in female than male home range size the year after shearing. In contrast, in roe deer disturbance associated with capture temporarily reduced activity levels and had a stronger effect on males than females (Morellet et al., Reference Morellet, Verheyden, Angibault, Cargnelutti, Lourtet and Hewison2009). We found no correlation between the proportion of females with young and the home range size.
The effects of capture on the movement patterns of guanacos in the Reserve lasted < 1 month, and probably only a few days. It was not possible to differentiate the effects of capture and shearing from seasonal or inter-annual changes in movement. However, the reduction in home range size 1 month after shearing was probably the result of reduced movement associated with the calving season and termination of the winter migration, as a similar reduction was also observed 1 year after shearing.
The increase in group density and individual density 1 month after capture and shearing indicates that the dispersal effect was reversed. However, the mean group size decreased, which is consistent with the formation of reproductive groups (or family groups) at this time of year (November–December), which often do not exceed 10–12 individuals.
At the sheep ranch, round-up, capture and shearing of guanacos did not affect the population parameters analysed nor result in increased movement of guanacos. During 4 years we studied 660 guanaco groups in post-shearing surveys and recorded only one shorn guanaco outside the 5,000-ha paddocks in which the animals were shorn (P. Carmanchahi., unpubl. data). At this site guanacos seemed to exhibit similar behaviour in response to disturbance, moving away from the shearing corral, but remained within the paddock.
Territorial stability following shearing was also observed in radio-collared guanacos on a private ranch in Patagonia (Rey et al., Reference Rey, Novaro, Sahores and Guichón2012b) and in vicuñas Vicugna vicugna in north-west Argentina (Arzamendia & Vilá, Reference Arzamendia and Vilá2012).
Effects on spatial distribution
Studies have shown that spatial distribution in mammals is influenced by (1) physiological factors, such as body size (Swihart et al., Reference Swihart, Slade and Bergstrom1988), sex and age (Relyea et al., Reference Relyea, Lawrence and Demarais2000), and reproductive status (Bertrand et al., Reference Bertrand, DeNicola, Beissinger and Swihart1996), (2) ecological factors, such as intra- (Riley & Dood, Reference Riley and Dood1984) and inter-specific competition (Loft et al., Reference Loft, Kie and Menke1993), and trophic level (Harestad & Bunnell, Reference Harestad and Bunnell1979), and (3) environmental factors, such as season, (Nicholson et al., Reference Nicholson, Bowyer and Kie1997) and the availability of forage (Relyea et al., Reference Relyea, Lawrence and Demarais2000) and water (Bowers et al., Reference Bowers, Welch and Carr1990). However, few studies have investigated the short-term effects of anthropogenic activities, such as management of wildlife, on spatial distribution.
At the sheep ranch, spatial distribution of guanacos appeared to be affected by management, as guanaco density decreased near the shearing corral. Guanacos were concentrated at sites far from the shearing corral but did not leave the paddock. At the Reserve, however, there was no clear effect of management on the spatial distribution of guanacos. The absence of physical barriers within the Reserve allows animals to move more freely away from the site of the corral and camp, and therefore we did not observe differences in the pattern of spatial distribution for each distance interval before and after assembly of the camp.
Conclusion and management implications
The methods used for capture and handling of a wild species may determine the effect of such management interventions on the physiological, behavioural and population parameters of the species. If capture and handling are carried out in accordance with animal welfare standards, physiological stress (Carmanchahi et al., Reference Carmanchahi, Ovejero, Marull, López, Schroeder and Jahn2011), behavioural stress (Taraborelli et al., Reference Taraborelli, Ovejero, Schroeder, Moreno, Gregorio and Carmanchahi2011) and mortality after shearing (Carmanchahi et al., Reference Carmanchahi, Ovejero, Marull, López, Schroeder and Jahn2011) can be reduced. However, we found that capture and handling of guanacos had short-term spatial and temporal effects on population parameters, with our results suggesting a dispersion effect among guanacos that were captured and shorn. In an open area groups moved away from the area where shearing took place, with shorn individuals moving > 13 km the first day. Sedentary populations also dispersed in response to handling but temporal patterns of individual and group density and group sizes were not modified within the managed paddock. In contrast, the spatial pattern was modified, with guanacos concentrated in areas far from the capture corral.
Our results support the hypothesis that the effects of capture and shearing of wild guanacos differ in sedentary and migratory populations. The adaptive importance of migratory movements is to guarantee favourable conditions for the existence and reproduction of the population. The delineation of grazing paddock by wire fences in Patagonia has led to a disruption of migratory paths of guanacos and the establishment of sedentary populations. A similar effect was observed in pronghorns Antilocapra americana in Texas, where migration appears to have been truncated by fences (Hailey & DeArment, Reference Hailey and DeArment1969). This significant anthropogenic factor has implications for the survival of guanacos because it may prevent animals from searching for new places with better environmental conditions. Fencing may also increase the local density of both livestock and wildlife in an area, preventing the natural rotation of use of food resources and seasonal recovery of pastures, and leading to degradation of vegetation and starvation of animals (Boone & Thompson Hobbs, Reference Boone and Thompson Hobbs2004). Our results show that capture and shearing of wild guanacos disturb population parameters significantly but for a relatively short period. Specifically, we documented dispersion and group disruption, similar to the findings of Sarno et al. (Reference Sarno, González, Bonacic, Zapata, O'Brien and Johnson2009) for vicuñas, which if not minimized by appropriate management could have a negative effect on social composition and population dynamics. We recommend that management authorities do not allow pre- and post-partum round-up and shearing in the same year. Although we did not measure the effect of this activity on the mother–calf relationship we cannot rule out disruption, and therefore we recommend that after post-partum shearing mothers and their calves are reunited in corrals before release. The management of wild guanacos for live-shearing, if based on high standards of animal welfare and monitored closely, could contribute to the sustainable use and conservation of the species as well as to the socioeconomic development of the region.
Acknowledgements
This work was funded by CONICET PIP No. 11220100100386, ANPCyT-PICT 1305/2010 and PICT 34120/2005. We thank Cooperativa Payun Matrú members, park rangers, the government authorities of Mendoza province, and David Garrido. The project was approved by the Department of Renewable Natural Resources of Mendoza province (Resoluciones 117/09; 795/10, DRNR–Secretaría de Medio Ambiente), and the Wildlife Agency of Río Negro province. We thank Dr Heiko Wittmer and an anonymous reviewer for their comments, which helped improve this article.
Biographical sketches
Pablo Carmanchahi's research focuses on the eco-physiological and population effects of guanaco management, and the development of sustainable alternatives for production on arid land. Natalia Schroeder is interested in the ecological and anthropogenic factors affecting the spatial and temporal distribution of the guanaco. María José Bolgeri studies guanaco migration and predation on guanacos. Susan Walker focuses on landscape ecology and wildlife conservation. Martín Funes works on the conservation of Patagonian wildlife, and invasive species. Jodi Berg is interested in guanaco ecology and the use of dogs for conservation. Paula Taraborelli studies behavioural ecology, and the effects of management on guanaco behaviour. Ramiro Ovejero studies the physiological effects of ecological and anthropogenic factors. Pablo Gregorio is interested in the nutritional quality of guanaco diet and its influence on physiological and ecological processes. Pablo Moreno focuses on the effects of parasitism on physiological and ecological processes. Andrés Novaro focuses on wildlife hunting, predator–prey interactions and conservation.







