Introduction
The objective of a reintroduction is to re-establish a population of a species within its indigenous range (Seddon, Reference Seddon2010; IUCN/SSC, 2013); globally many reintroductions have taken place but the outcomes of these projects are variable (Soorae, Reference Soorae2008, Reference Soorae2010, Reference Soorae2011, Reference Soorae2013). A variety of tactics can be incorporated into a reintroduction process to improve the performance (e.g. survival, reproduction) and behaviour (e.g. settlement and dispersal) of the founder population post-release (Batson et al., Reference Batson, Abbott, Richardson, Armstrong, Hayward, Moro and Seddon2015). Other tactics can be used to manage the ecological risks associated with reintroductions, including quarantine to avoid detrimental disease and co-introductions of pathogens or parasites (Woodford, Reference Woodford2000). Aspects of a reintroduction that are focused at a population level are usually viewed independently from those focused on the ecosystem (Armstrong & Seddon, Reference Armstrong and Seddon2008). However, certain tactics can induce responses across these ecological levels, and improving our understanding of these could improve the quality and efficiency of reintroduction strategies.
The selection of release tactics is usually defined as a choice between a delayed release, when founders are housed in situ at the release site temporarily prior to release, and an immediate release, with no pre-release confinement (Parker et al., Reference Parker, Dickens, Clarke, Lovegrove, Ewen, Armstrong, Parker and Seddon2012). These are described as soft and hard release, respectively (Wanless et al., Reference Wanless, Cunningham, Hockey, Wanless, White and Wiseman2002; Mitchell et al., Reference Mitchell, Wellicome, Brodie and Cheng2011), but these terms are considered inappropriate unless the effect on the severity of transition into the recipient environment is known (Parker et al., Reference Parker, Dickens, Clarke, Lovegrove, Ewen, Armstrong, Parker and Seddon2012; Moseby et al., Reference Moseby, Hill and Lavery2014, Batson et al., Reference Batson, Abbott, Richardson, Armstrong, Hayward, Moro and Seddon2015). Delayed release can improve the probability of establishment by allowing founders to recover, acclimatize, establish social relationships and become familiar with their surroundings prior to release (Bright & Morris, Reference Bright and Morris1994; Gusset et al., Reference Gusset, Slotow and Somers2006; Mitchell et al., Reference Mitchell, Wellicome, Brodie and Cheng2011). However, adopting this approach can have a detrimental effect by increasing mortality, stress and injury, especially in wild animals (Christensen & Burrows, Reference Christensen, Burrows and Serena1994; Linklater et al., Reference Linklater, MacDonald, Flamand and Czekala2010; Richardson et al., Reference Richardson, Castro, Brunton and Armstrong2015). In other situations the release tactic used has no effect on the probability of establishment (Castro et al., Reference Castro, Alley, Empson, Minot and Serena1994; Lovegrove, Reference Lovegrove1996; Hardman & Moro, Reference Hardman and Moro2006), which makes immediate release preferable on the grounds of reduced cost (Hardman & Moro, Reference Hardman and Moro2006).
The variability of responses to release tactics inhibits the ability to make sweeping recommendations regarding the most appropriate approach when faced with uncertainty (Parker et al., Reference Parker, Dickens, Clarke, Lovegrove, Ewen, Armstrong, Parker and Seddon2012). However, some general recommendations are provided for certain reintroduction contexts, including the use of delayed releases for captive-bred birds, and immediate releases for wild birds, based on their familiarity and reaction to confinement (Jones & Merton, Reference Jones, Merton, Ewen, Armstrong, Parker and Seddon2012). The ability to make general recommendations will improve through the accumulation of experimental evidence, highlighting the value of conducting reintroductions within experimental frameworks to test the effectiveness of methodological variations (Armstrong et al., Reference Armstrong, Soderquist, Southgate and Serena1994; Moseby et al., Reference Moseby, Hill and Lavery2014; Kemp et al., Reference Kemp, Norbury, Groenewegen, Comer, Armstrong, Hayward, Moro and Seddon2015).
All translocations present a risk that novel organisms will be co-introduced to the recipient environment, and managing this risk should be a key consideration when developing translocation strategies (IUCN/SSC, 2013). Quarantine is often used to manage this risk, and is often conducted within specialist captive facilities that provide the required level of isolation (Woodford, Reference Woodford2000). Although quarantine is used primarily to manage ecological risks it can also induce biological, behavioural or physiological responses in founder populations; for example, exposing European rabbits Oryctolagus cuniculus to quarantine generally improves their body condition but causes females to abort reproduction (Calvete et al., Reference Calvete, Angulo, Estrada, Moreno and Villafuerte2005). As quarantine can affect the performance of translocated wildlife, these effects must be considered carefully when developing translocation strategies.
Many reintroductions include both ex situ quarantine and in situ confinement to obtain population and ecosystem benefits (e.g. McClelland & Gummer, Reference McClelland and Gummer2006; Cid et al., Reference Cid, Figueira, Mello, Pires and Fernandez2014; Kenyon et al., Reference Kenyon, Streicher, Loung, Tran, Tran, Vo and Cronin2014). However, in certain situations it may be possible to use ex situ captivity to achieve multiple benefits, including managing ecological risk and improving the probability of establishment; for example, wild Canada lynx Lynx canadensis showed an improved rate of post-release survival after being held temporarily in ex situ captivity (Devineau et al., Reference Devineau, Shenk, Doherty, White and Kahn2011), with this period presumably also presenting the opportunity to conduct quarantine if required. The ability to use a single period of confinement to serve both benefits has obvious attractions, as multiple confinement periods invariably increase the financial cost (Karesh, Reference Karesh1993; Henri et al., Reference Henri, Milne and Shah2004).
We investigated whether housing wild eastern bettongs Bettongia gaimardi in ex situ captivity for 95–345 days prior to release influences their body mass, survival and pouch occupancy during the initial 1.5 years post-release, compared with those exposed to an immediate release. Based on our results we provide practical recommendations regarding the use of ex situ captivity in subsequent reintroductions. We also tested whether the performance of the founders differed from our pre-release expectations, to assess the effect of the reintroduction and to evaluate post-release establishment. This study focused on the founder population at Mulligans Flat Woodland Sanctuary, in the Australian Capital Territory, released during 2012. This reintroduction represents the first attempt to re-establish eastern bettongs on the Australian mainland following a 100-year absence (Short, Reference Short1998), and is a component of a large-scale experiment aiming to restore biological integrity and ecological function to a critically threatened woodland community (Manning et al., Reference Manning, Wood, Cunningham, McIntyre, Shorthouse, Gordon and Lindenmayer2011; Shorthouse et al., Reference Shorthouse, Iglesias, Jeffress, Lane, Mills and Woodbridge2012).
Study areas and species
Tidbinbilla Nature Reserve is located in rural Australian Capital Territory and is owned and operated by the territory government. The Reserve is a certified member of the Zoo and Aquarium Association and operates captive breeding programmes for various threatened species, including northern corroboree frogs Pseudophryne pengilleyi and southern brush-tailed rock-wallabies Petrogale penicillata. A permanent insurance population of eastern bettongs was also established at the Reserve, which housed the delayed-release group during the pre-release confinement period. Bettongs were predominantly housed within 2.6–9.4 ha enclosures, with small groups (< 5) housed in smaller enclosures (0.5–1 ha) during an initial 30-day quarantine and during trials. The composition of the groups within each enclosure was managed to ensure that reproduction could only occur among individuals from different regions in Tasmania (Fig. 1). A specialized on-site veterinary centre was used to conduct all health assessments (Portas et al., Reference Portas, Fletcher, Spratt, Reiss, Holz and Stalder2014). All enclosures were protected by electrified fences and were not accessible by the public. Food (fruits, vegetables, nuts, seeds and proteins) and water were provided daily ad libitum. All enclosures included natural vegetation suitable for bettongs to make diurnal nests and for natural foraging behaviour.
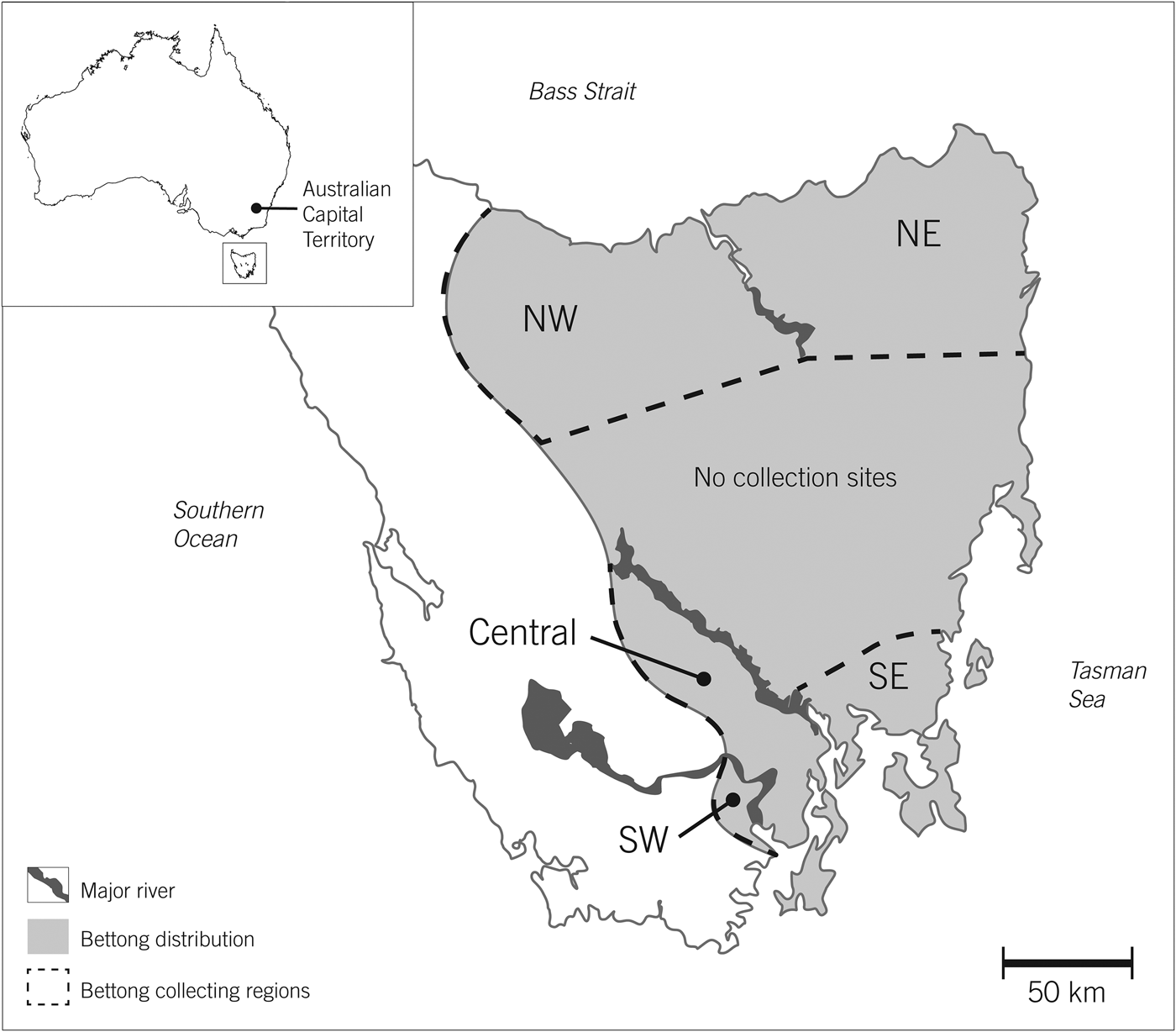
Fig. 1 Regions of Tasmania where eastern bettongs Bettongia gaimardi in free-ranging populations were trapped for reintroduction in Australian Capital Territory. As a precaution, each region was assumed to be genetically isolated by geographical barriers (e.g. major rivers).
Mulligans Flat Woodland Sanctuary is a publicly accessible area within Mulligans Flat Nature Reserve, adjacent to the northern suburbs of Canberra, and is co-managed by the Woodlands and Wetlands Trust and the Australian Capital Territory government. It is c. 60 km from Tidbinbilla Nature Reserve. The Sanctuary encompasses 485 ha of critically threatened mixed yellow-box Eucalyptus melliodora and Blakely's red gum Eucalyptus blakelyi grassy woodland (McIntyre et al., Reference McIntyre, Stol, Harvey, Nicholls, Campbell and Reid2010), enclosed by a barrier fence against foxes, cats and dogs, which have been eradicated from the internal area. The Sanctuary is considered an outdoor laboratory and is the location of the Mulligans Flat–Goorooyarroo Woodland Experiment (Manning et al., Reference Manning, Wood, Cunningham, McIntyre, Shorthouse, Gordon and Lindenmayer2011; Shorthouse et al., Reference Shorthouse, Iglesias, Jeffress, Lane, Mills and Woodbridge2012). The bettong population is treated as wild, with no husbandry management or supplementary resources provided. Bettongs have complete access to the Sanctuary, except for 12 1-ha sites that are fenced to facilitate assessment of the ecological effect of bettong diggings.
Eastern bettongs (also known as Tasmanian bettongs) are nocturnal, ground dwelling, mycophagous marsupials that occupy various woodland and forest habitats (Taylor, Reference Taylor1993a,Reference Taylorb; Johnson, Reference Johnson1994). Females reach sexual maturity at c. 9 months of age and are capable of near-continuous breeding (Rose, Reference Rose1987). Once common throughout eastern mainland Australia, their distribution is now restricted to eastern Tasmania (Fig. 1) and the species is categorized as Near Threatened on the IUCN Red List (Menkhorst, Reference Menkhorst2008). Disease transmission from feral cats has been implicated as a cause of a recent population decline (Fancourt, Reference Fancourt2014). Bettongs dig soil when foraging and are therefore considered to be ecosystem engineers, and their reintroduction may help to re-establish diminished ecological processes (Fleming et al., Reference Fleming, Anderson, Prendergast, Bretz, Valentine and Hardy2014; Manning et al., Reference Manning, Eldridge, Jones, Armstrong, Hayward, Moro and Seddon2015).
Methods
The translocation process
Sixty adults (19 male, 41 female) and their 28 pouch young were translocated from Tasmania to the Australian Capital Territory in four collection events during July 2011–September 2012 (Table 1). Bettongs were collected from wild populations from five geographical areas in Tasmania to increase genetic diversity (Fig. 1). Subadults, females carrying furred pouch young, and females with elongated teats were excluded from the translocation. A female-biased sex ratio was established to increase post-translocation population growth, and the pouches of females carrying pouch young were taped to prevent ejection. Once selected for translocation each individual was weighed and administered diazepam to act as a mild sedative, before being transported by road and air to Tidbinbilla Nature Reserve, where they arrived within 18 hours of acquisition. A second dose of diazepam was administered immediately before air transportation. Upon arrival each individual was anaesthetized, fitted with a passive integrated transponder tag, and given a full health assessment by a qualified veterinarian, which included measurements of body mass, pes (foot) length, tail width, head length and ectoparasite load, and classifications of body condition (using a subjective assessment of fat stores around hips), tooth wear and coat condition. Rectal, urogenital, conjunctival and nasal tract swabs and blood samples were collected to evaluate pathogen history and endoparasite load, and ear biopsies were collected for genetic analyses. The head length and sex of pouch young were also assessed. No food or water was provided during the translocation process but saline was administrated intravenously if required. Portas et al. (Reference Portas, Fletcher, Spratt, Reiss, Holz and Stalder2014) provide further details regarding the translocation process and health assessments.
Table 1 The reintroduction history of the founder population of eastern bettongs Bettongia gaimardi, with ID, date of acquisition, date of release, sex, release group, condition at release, mortality, origin (Fig. 1), and the number of times each individual was trapped during acquisition, release, and 1–60, 61–180, 181–360 and 361–540 days post-release.
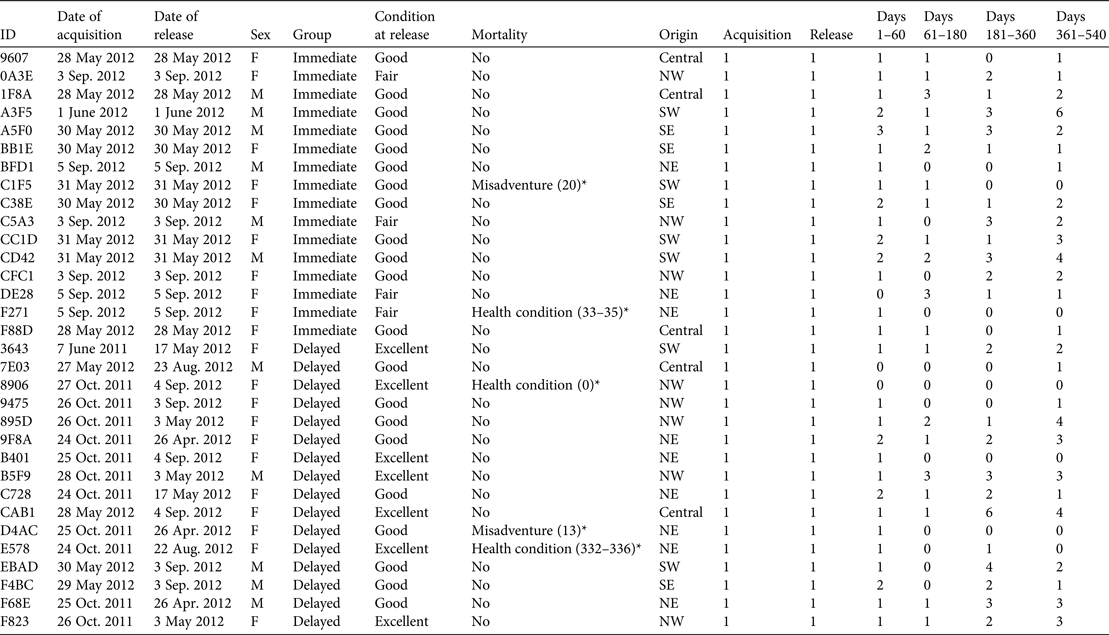
* The number in parentheses indicates the timing of mortality in terms of number of days post-release.
Upon arrival each bettong was assigned at random to a population (Tidbinbilla Nature Reserve or Mulligans Flat Woodland Sanctuary) but those with health conditions were kept permanently at the Reserve. Pouch young stayed with their mothers throughout the translocation. Twenty-eight adults were assigned to the permanent captive population at the Reserve. The remaining 32 adults were assigned to the wild population at the Sanctuary, with 16 (11 female) in the delayed-release group (i.e. housed at the Reserve prior to release at the Sanctuary), and 16 (10 female) in the immediate-release group. Following the completion of the initial health assessments those assigned to the delayed-release group were released into small enclosures at the Reserve for a 30-day quarantine period. Following a post-quarantine health assessment members of this group were moved to the large enclosures, where they remained until their transfer to the Sanctuary. Upon completion of the 95–345 day confinement period bettongs were transferred to the Sanctuary in similar sized groups as the immediate-release group (Table 1), and released at similar times. Members of the immediate-release group were transferred and released at the Sanctuary following the completion of the heath assessment at the Reserve on the day of translocation. All immediate releases occurred within 24 hours of initial acquisition in Tasmania.
Post-release monitoring
Thirty-one founders were fitted with VHF (V5C_161C; Sirtrack, Hawkes Bay, New Zealand) or global positioning system (GPS)/VHF radio collars (Q4000E; Telemetry Solutions, Walnut Creek, USA) when released. One individual was not collared because of a neck injury. Each collar weighed 28–32 g, which is < 2.5% of the body mass of the lightest individual released. The collars transmitted a continuous VHF pulse, and a mortality signal was activated following 12 hours without movement. The post-release survival of each individual was monitored daily for 1 month post-release, and thereafter at least weekly until the collar was removed after 1 year. If a mortality signal was detected the collar was located immediately to determine the cause. On one occasion a collar was removed because of injury, and four collars detached accidentally. Three of the detached collars were reattached before the completion of the monitoring period. Necropsies were conducted on all deceased individuals (Portas et al., Reference Portas, Fletcher, Spratt, Reiss, Holz and Stalder2014).
Post-release health assessments were scheduled to occur at 1, 3, 6 and 12 months post-release but the timing and frequency varied because of logistical constraints (Table 1). To trap bettongs for a scheduled health assessment we radio-tracked each individual of interest to its daytime nest and deployed six traps in close proximity. The health assessment included measurements of body mass, pes length and tail width, assessment of body condition, and measurement of the head length of pouch young. The assessments were conducted without sedation but with procedures in place to minimize handling time, which was generally < 10 minutes. The pouches of females carrying unfused pouch young were taped to reduce the risk of pouch ejection (the tape detaches within a few hours). Individuals were released at the point of capture upon completion of the health assessment. When non-target individuals were captured they were either given a full health assessment or were weighed and released, depending on the proximity to their scheduled health assessment. In total, 218 capture events were recorded during the monitoring period.
Statistical methods
All statistical analyses were conducted using SPSS v. 22 (IBM, Armonk, USA), with significance assumed at P < 0.05.
Body mass We used body mass as a proxy for body condition (sensu Moseby et al., Reference Moseby, Hill and Lavery2014). We opted not to use a body condition index (e.g. Hardman & Moro, Reference Hardman and Moro2006) because of the lack of correlation between pes length and body mass in our data (R2
< 0.1). The body mass of females with occupied pouches was adjusted by subtracting the estimated mass of the pouch young. This was calculated using the quadratic equation for estimating the age of a pouch young from its head length and an exponential equation to estimate its mass from its estimated age, as described by Rose (Reference Seddon, Armstrong and Maloney1989). We excluded the body mass of females carrying pouch young from the analysis if the head length of the pouch young was not recorded. The records were divided into the following periods: acquisition, data collected during translocations from Tasmania; release, data collected when individuals were released at the Sanctuary (synonymous with acquisition for the immediate-release group); days 1–60, data collected 1–60 days post-release; days 61–180, data collected 61–180 days post-release; days 181–360, data collected 181–360 days post-release; days 361–540, data collected 361–540 days post-release. To minimize the effect of repeated measures we used the mean body mass of any individual captured multiple times within a period, which reduced the dataset to 143 samples. We compared the body mass of the two groups using a linear mixed model with time and group as factors (using a compound symmetry correlation structure), with release as the starting point. We conducted randomization tests to assess whether body mass within the two groups was different within each period. This process was similar to that used by Moseby et al. (Reference Moseby, Hill and Lavery2014). We did not differentiate between sexes because of the lack of sexual dimorphism (Rose, Reference Rose1989; Claridge et al., Reference Claridge, Seebeck and Rose2007). We compared the post-release body mass of the entire population against our pre-release expectation, using a randomization test. Our expectation was set according to the body mass at acquisition (
![]() $ {\tilde{x}}=1,629 \pm {\rm SD} 176 {\rm g})$
.
$ {\tilde{x}}=1,629 \pm {\rm SD} 176 {\rm g})$
.
Pouch occupancy Pouch occupancy was assessed by visually inspecting the pouches of females during health assessments. A pouch was considered occupied if a pouch young was observed in the pouch or in the trap with the adult. The data were organized into the periods described above, with samples excluded if the pouch young had been recorded previously, based on the expected growth rate and a 106-day pouch life (Rose, Reference Rose1989). It was possible for multiple pouch young to be recorded from a single female within a period when pouch young were replaced between health assessments. The proportions of pouch occupancy of the two groups were compared for each period using Fisher's exact test. This approach was also used to assess whether post-release pouch occupancy for the entire population differed from our pre-release expectation, which was set at 0.71, representing the proportion observed at acquisition. We confirmed that all delayed-released females had access to potential mates at the Reserve within 106 days of release, to ensure that pouch inactivity was not attributable to lack of mating opportunities.
Survival No meaningful statistical comparison of survival between the two groups was possible because of the low number of mortalities, and therefore only descriptive accounts are presented. We tested whether the mortality rate observed during the first year post-release differed from the expected rate of 0.2 per annum using Fisher's exact test. The expected mortality rate was based on the maximum life expectancy of 6 years (Rose, Reference Rose1987), and the ages of founders randomly falling between 1 and 6 years when released. This assumption was used because the ages at acquisition could not be estimated accurately, with the minimum age being based on the exclusion of non-mature individuals at acquisition. The analysis was restricted to the first year post-release because the status of all individuals was known following completion of this period, although some of the evidence for this was outside the data set used during this study.
Results
The linear mixed effect model indicated there was no significant difference between the body mass of the two groups (F 1,30 = 0.161, P = 0.691). However, the body mass of founders was influenced by time (F 4,88 = 4.674, P = 0.002), and there was a significant interaction between time and treatment (F 4,88 = 6.999, P < 0.001). These results reflect that the delayed-release group was heavier when released, and the extra mass was lost soon after release, before stabilizing, whereas the immediate-release group maintained consistent body mass across the monitoring period. The randomization tests confirmed that the only significant difference between the two groups was at release (P < 0.001), although the difference approached significance at acquisition (P = 0.1). Overall, the post-release body masses recorded at the Sanctuary exceeded our initial expectation (P = 0.005), indicating that the body mass of the whole population increased significantly post-release (Fig. 2).
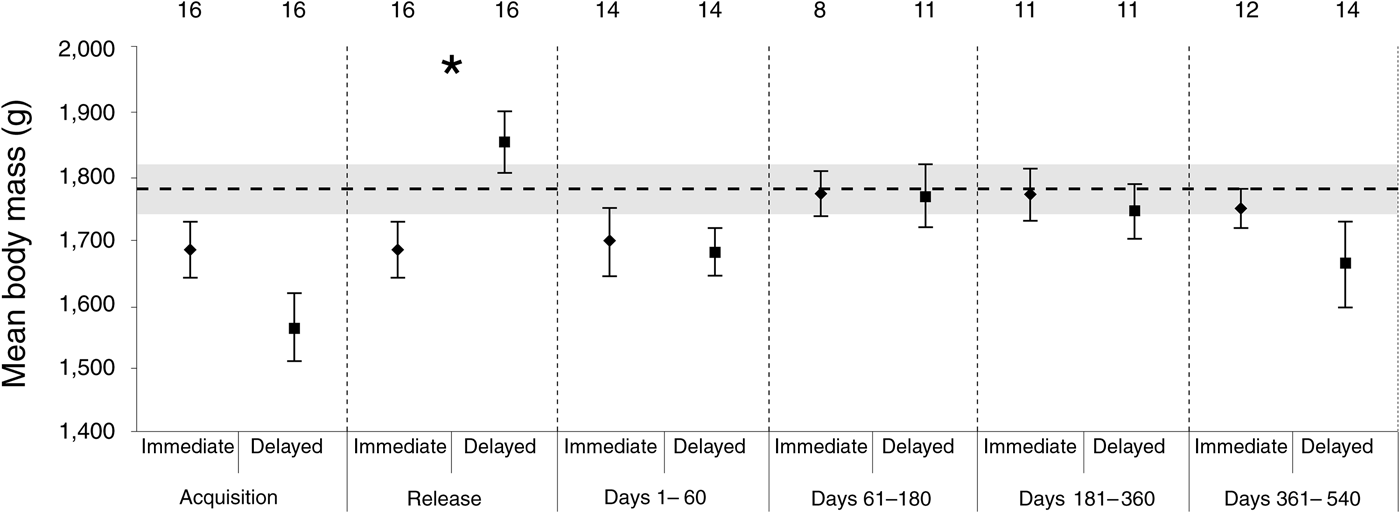
Fig. 2 The mean body mass of bettongs in each release group within six sampling periods: acquisition, release, and 1–60, 61–180, 181–360 and 361–540 days post-release. The numbers above the data points represent the number of individuals sampled, and the asterisk represents a significant difference between the groups. Error bars represent ± 1 SE. The horizontal line represents the expected body mass based on that recorded at acquisition, with the shaded area representing ± 1 SE.
The proportion of pouch occupancy was greater in the immediate-release group compared to the delayed-release group at release (P = 0.03), with no other significant between-group differences occurring within any other period (Fig. 3). Overall, the rate of post-release pouch occupancy differed significantly from the expected rate (P = 0.01), indicating that the reproductive activity of females was higher at the Sanctuary compared to the source populations in Tasmania. Two pouch young were known to be lost between sampling events prior to the expected 106 day pouch life, and pouch occupancy was recorded in all surviving females within 6 months of release.
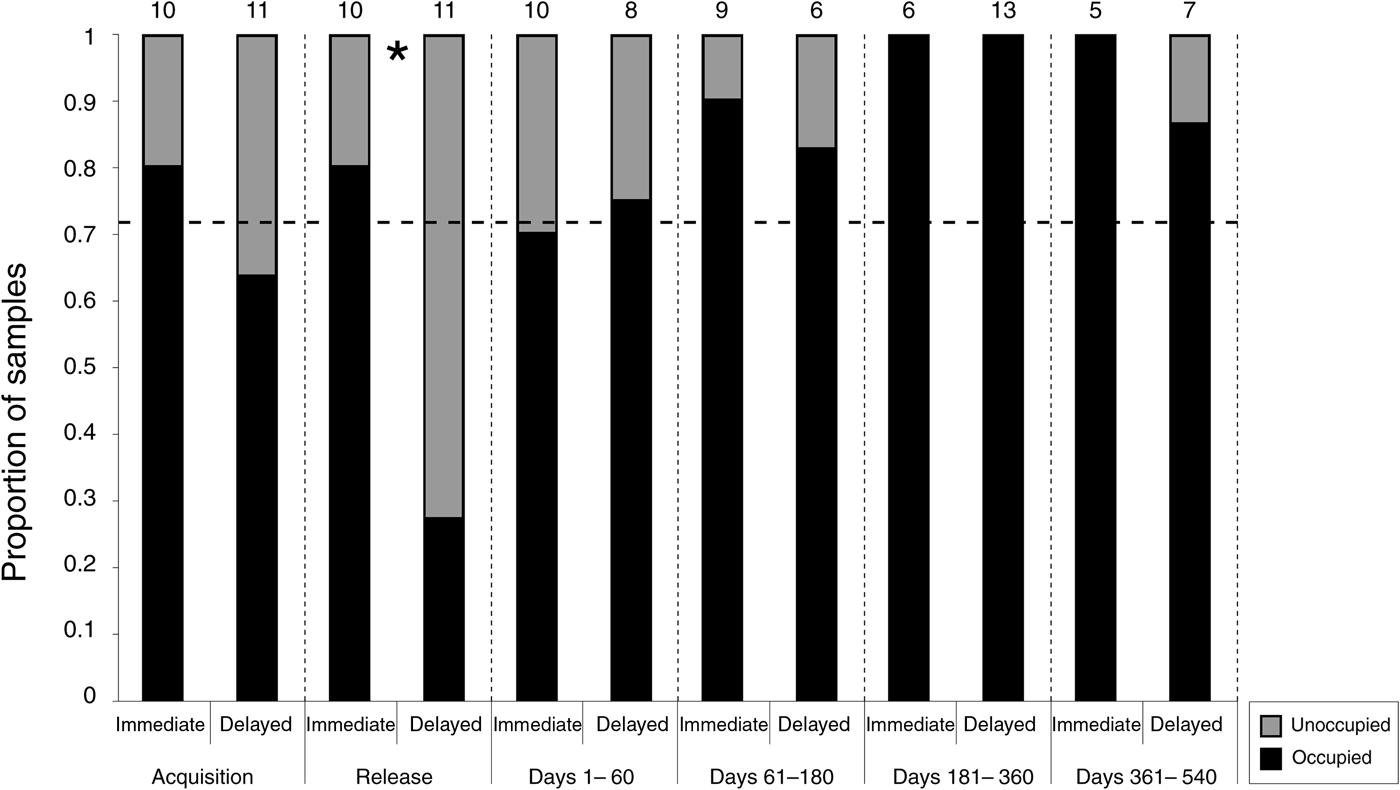
Fig. 3 The rate of pouch occupancy recorded in each group within the six sampling periods. The numbers above the bars represent the number of individuals sampled, and the asterisk represents a significant difference between the groups. The horizontal line represents the expected rate of pouch occupancy based on the rate recorded at acquisition.
Five mortalities were recorded during the monitoring period. All deceased bettongs were female; three were members of the delayed-release group (Table 1). Necropsies confirmed that three of the mortalities resulted from pre-existing health conditions (two in the delayed-release group), with the remaining deaths being attributed to misadventure. The timing of two of the mortalities may have been influenced by the reintroduction process, given the temporal proximity to release: a member of the delayed-release group did not recover from being anaesthetized on the day of release, and a member of the immediate-release group died c. 1 month post-release. Overall, the mortality rate observed during the first year post-release was 0.16, which did not differ significantly from the expected mortality rate of 0.2 (P = 1).
Discussion
Our results suggest that exposing founders to ex situ captivity did not influence the body mass, pouch occupancy or survival of the founder group within any period post-release for the bettongs released at the Sanctuary. This is despite the delayed-release group being significantly heavier (+ 10%) and having a lower rate of pouch occupancy (27 vs 80%) than the immediate-release group when released. Overall, this indicates pre-release captivity does not represent a viable release tactic for improving the performance of founders post-release, unless it induces a positive behavioural response, which was not assessed in this study.
The lack of a significant effect on post-release survival is consistent with the results of similar studies involving translocated macropods (family Macropodidae); for example, implementing delayed and immediate releases did not affect post-release survival in burrowing bettongs Bettongia lesueur, greater bilbies Macrotis lagotis (Moseby et al., Reference Moseby, Hill and Lavery2014) or banded Lagostrophus fasciatus and rufous hare-wallabies Lagorchestes hirsutus (Hardman & Moro, Reference Hardman and Moro2006). As these studies involved wild and captive-bred macropods, it appears that the life history of founders does not alter the survival response to various release tactics, which contrasts with the general trend observed in birds, whereby survival is generally higher when captive-bred birds are exposed to a delayed release, and the converse is true for certain species of wild birds (Mitchell et al., Reference Mitchell, Wellicome, Brodie and Cheng2011; Jones & Merton, Reference Jones, Merton, Ewen, Armstrong, Parker and Seddon2012; Richardson et al., Reference Richardson, Castro, Brunton and Armstrong2015). As many of the macropod studies have been conducted in the absence of exotic predators (e.g. this study; Moseby et al., Reference Moseby, Hill and Lavery2014), and involved small experimental groups (e.g. Hardman & Moro, Reference Hardman and Moro2006; Moseby et al., Reference Moseby, Hill and Lavery2014), the effect of release tactics on predation vulnerability needs to be assessed before robust conclusions regarding reintroductions to wild sites can be drawn.
Our results suggest that captivity had a negative effect on reproduction, although near-continuous breeding has been achieved in another captive population of eastern bettongs (Rose, Reference Rose1987). The variability of captive pouch occupancy may indicate that reproduction is primarily affected when wild bettongs are temporarily exposed to captivity, or that there is a specific cause at Tidbinbilla Nature Reserve, with obesity, diet, stress and human-determined mate-choice providing possible explanations (Kleiman et al., Reference Kleiman, Thompson and Baer2010; Michel & Bonnet, Reference Michel and Bonnet2012). The reduction of pouch occupancy at release needs to be considered when developing reintroduction strategies for eastern bettongs because it will increase the lag time to post-release recruitment. However, as every surviving female was observed to be reproductively active within 6 months of release, the initial reduced proportion of pouch occupancy is unlikely to affect the long-term genetic viability (Jamieson & Lacy, Reference Jamieson, Lacy, Ewen, Armstrong, Parker and Seddon2012).
The body mass advantage of the delayed-release group at release was not maintained, with no significant differences detected post-release. Moseby et al. (Reference Moseby, Hill and Lavery2014) observed a similar trend in burrowing bettongs, although the delayed-release group was still relatively heavier 2 weeks after release, partly because the immediate-release group lost weight during that period. Although an immediate weight loss was not detected in the immediate-release animals in our study, it may have occurred without being detected, given the frequency of trapping events. Overall, it appears that the body mass of translocated bettongs (eastern and burrowing) is determined primarily by environmental surroundings, and that the relative body mass at release has only a short-term effect. This also suggests that temporarily exposing wild bettongs to captivity does not influence their ability to acquire resources once released back into the wild.
The body mass and rate of pouch occupancy in the founder group post-release exceeded our expectation, whereas post-release survival was consistent with the expected rate. However, as 80% of the mortalities recorded appear to have been influenced by the translocation process or post-release monitoring, survival at the Sanctuary could also be considered to have exceeded the expected rate. The performance of the founder group reflects the suitability of the habitat, low levels of competition, and absence of exotic predators at the Sanctuary, and provides evidence that the founder group transitioned successfully through the establishment phase of a reintroduction (Armstrong & Seddon, Reference Armstrong and Seddon2008; IUCN/SSC, 2013). This is also supported by the recruitment of new individuals at the Sanctuary. Given the favourable conditions at the Sanctuary it is likely that the body mass and performance recorded in the founder group were near-optimal for a wild population, which provides a useful comparison to evaluate the condition of other populations.
The lack of a significant biological response to varying release tactics is consistent with the general outcomes of other studies involving reintroduced macropods, using in situ captivity for delayed release (Hardman & Moro, Reference Hardman and Moro2006; Moseby et al., Reference Moseby, Hill and Lavery2014). In addition to the effects on body mass, survival and reproduction, release tactics were also found to have no effect on settlement or dispersal in greater bilbies (Moseby et al., Reference Moseby, Hill and Lavery2014) or banded and rufous hare-wallabies (Hardman & Moro, Reference Hardman and Moro2006) despite influencing settlement in burrowing bettongs (Moseby et al., Reference Moseby, Hill and Lavery2014). As delayed release did not provide a significant establishment benefit we would recommend immediate release to increase resource efficiency if pre-release quarantine was not required. This conclusion is consistent with the prediction of the conceptual model presented by Moseby et al. (Reference Moseby, Hill and Lavery2014), based on the behavioural characteristics (sociality, site fidelity and ranging) of eastern bettongs and the environmental characteristics (fencing and predation risk) of Mulligans Flat Woodland Sanctuary.
Despite the lack of significant effects detected in macropod studies, the popularity of delayed releases appears to be increasing (Clayton et al., Reference Clayton, Pavey, Vernes and Tighe2014). This suggests that the designs of these reintroductions are based on perceived benefits rather than experimental evidence, which is a common feature of reintroductions (Parker et al., Reference Parker, Dickens, Clarke, Lovegrove, Ewen, Armstrong, Parker and Seddon2012). However, implementing a delayed release can provide a number of non-biological benefits. During this reintroduction the delayed release facilitated quarantine, ecological risk assessments (Portas et al., Reference Portas, Fletcher, Spratt, Reiss, Holz and Stalder2014), and equipment trials prior to release. The use of both release tactics within a structured framework spread the risk of failure by exposing founders to various methods, and facilitated experimental investigation of the responses to these variations. The delayed release also provided an opportunity for the bettongs that were translocated from Tasmania in poor condition to increase their body mass prior to release. Although many of the non-biological benefits could have been provided by in situ confinement, the use of ex situ captivity avoided the need to build new infrastructure, and the delayed-release group could be managed by professional staff as part of the daily operations at the Reserve.
We acknowledge that the strength of our statistical analyses is restricted by the small number of individuals, which is common in reintroduction biology (Seddon et al., Reference Seddon, Armstrong and Maloney2007). We also accept that the probability of success was high because of the lack of predators at the Sanctuary, and the barrier to dispersal (Short et al., Reference Short, Bradshaw, Giles, Prince and Wilson1992; Clayton et al., Reference Clayton, Pavey, Vernes and Tighe2014). However, low-risk reintroduction often represents the most appropriate environment to test the effectiveness of various methodologies, because predation and dispersal can mask subtle effects. The results of such experiments can then be used to develop new hypotheses and improve the quality of reintroduction strategies for releases into higher-risk environments. One of the strengths of this study is that it assessed the responses to release tactics over a prolonged period, which is sometimes essential to detect an effect (e.g. Richardson et al., Reference Richardson, Castro, Brunton and Armstrong2015).
Based on our results we recommend selecting release tactics based on evaluations of financial cost and ecological risk rather than the assumed effect on establishment. However, effects on stress, settlement, dispersal and vulnerability to predation need to be assessed before a robust conclusion can be drawn. If the risk of detrimental co-introduction is considered high in subsequent reintroductions, we advocate the use of a delayed release involving ex situ captivity as an appropriate form of quarantine, because of its minimal effect on establishment probabilities. We also recommend this approach when these ecological risks are unknown, as a precaution. However, if the ecological risks are considered low then an immediate release should be used to maximize cost efficiency.
Acknowledgements
The translocation was carried out under license from the Tasmanian Department of Primary Industries, Parks, Water and Environment (DPIPWE) using procedures approved by their associated Ethics Committee (AEC Project 18/2010–2011). We thank DPIPWE for their support. The post-reintroduction procedures were approved by the Australian National University Animal Experimentation Ethics Committee (ethics protocol A2011/017). WB was supported by a PhD scholarship funded through an Australian Research Council Linkage Grant (LP110100126). ADM was supported by an Australian Research Council Future Fellowship (FT100100358). This project was conducted as part of the Mulligans Flat–Goorooyarroo Woodland Experiment. We thank Helen Crisp, Jenny Newport, Nicola Munro, Tim Portas, Claire Wimpenny, Scott Ryan, David Dobroszczyk, Elyce Fraser, Ani Kunz, Andrea Reiss, Nick Mooney, Matthew Pauza, Peter Mills, Daniel Iglesias, Grant Woodbridge, John Lawler, Stuart Jefferies, David Shorthouse, Ross Cunningham, Jeff Wood, Christopher Johnson and Margret Kitchin for their assistance during the project. We thank Bob Forrester for his advice on statistics, and two anonymous reviewers for their valuable feedback.
Biographical sketches
William Batson's research focuses on the reintroduction biology of Australian and New Zealand fauna populations. Iain Gordon's research interests include the behaviour, ecology, management and environmental impacts of herbivore populations. He also has an interest in the practical and social aspects of ecological restoration. Donald Fletcher's research interests include the environmental impacts, management, conservation and ecology of native Australian macropods, and the restoration of Australian woodlands. Adrian Manning's research interests incorporate many aspects of conservation biology and ecological restoration. He has a special interest in the management and restoration of Australian woodlands.






