The blond titi monkey Callicebus barbarabrownae (Plate 1) lives in dry forest fragments in the caatinga (tropical thorn scrub and deciduous forest) in north-east Brazil. The colonization of this region and the widespread destruction of its vegetation began in the early 1500s and the caatinga forests have been largely destroyed, reduced to widely dispersed, small, degraded patches surrounded by scrub, barren pasture and crops (Coimbra-Filho & Câmara, Reference Coimbra-Filho, Câmara and de1996). When Hershkovitz (Reference Hershkovitz1990) described C. barbarabrownae it was known from just three localities in the state of Bahia: Lamarão (Table 1, Fig. 1: locality 40), Formosa (locality 41), and Bandeira de Mello (locality 39). Marinho-Filho & Veríssimo (Reference Marinho-Filho and Veríssimo1997) reported a specimen from Mirorós, Bahia (locality 38), which extended the range to the west, and indicated that the western limit was the Rio São Francisco. It has been categorized as Critically Endangered on the IUCN Red List since 1996 based on these four localities and a general understanding of the widespread destruction of the caatinga (Veiga et al., Reference Veiga, Printes, Rylands, Kierulff, de Oliveira and Mendes2008). Here we report a 2004–2005 survey of C. barbarabrownae in the states of Bahia, Sergipe and Alagoas that was designed to obtain a better understanding of the species’ habitat preferences and the extent of its geographical range and to verify its conservation status.

Fig. 1 Occurrence localities (see details in Table 1) of the blond titi monkey Callicebus barbarabrownae. The inset indicates the location of the main map in north-east Brazil.

Plate 1 Blond titi monkey Callicebus barbarabrownae family group (photograph: Antônio Estrela).
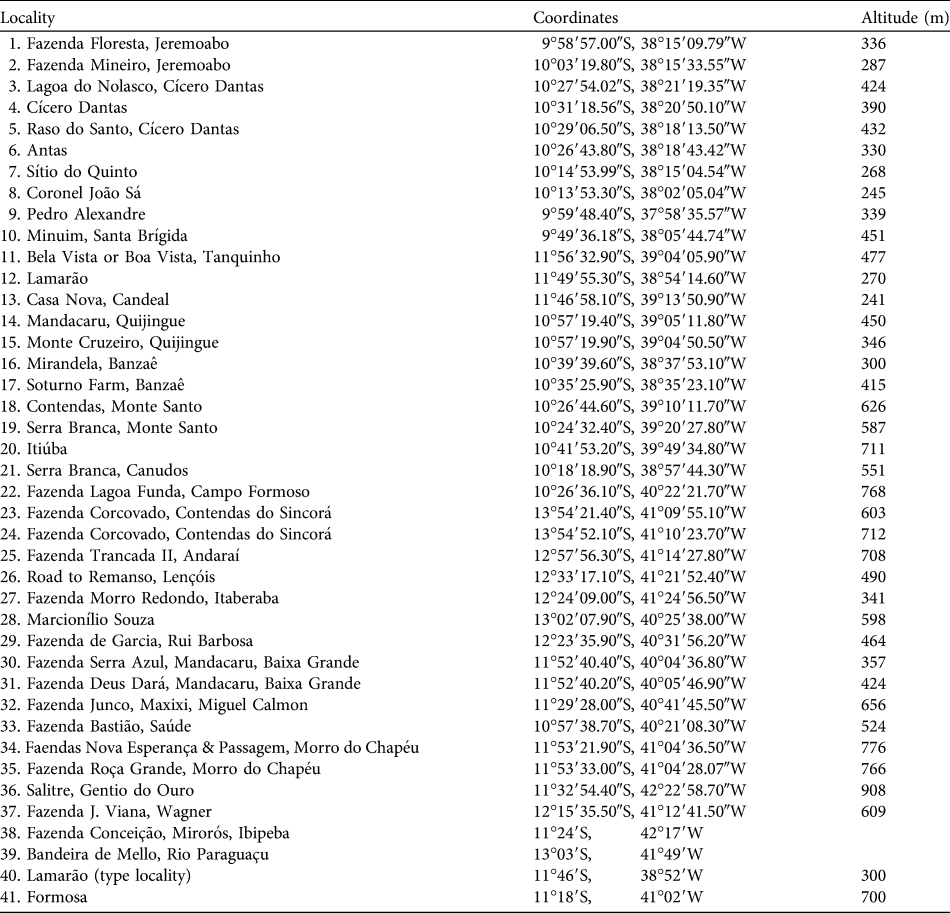
Table 1 Locality records of Callicebus barbarabrownae (see numbers in Fig. 1) obtained in this study (localities 1–37) and by Marinho-Filho & Veríssimo (Reference Marinho-Filho and Veríssimo1997; locality 38), and evidenced by specimens in the British Museum (Natural History), London, UK (localities 39–40; Napier, Reference Napier1976) and the Field Museum of Natural History, Chicago, USA (locality 41; Hershkovitz, Reference Hershkovitz1990).
From June 2004 to May 2005 (130 days of field research) we surveyed 353,925 km2 of the caatinga between the Rios São Francisco and Jequitinhonha in Bahia, including Sergipe and the deciduous and transitional deciduous to humid forests of Alagoas. We surveyed forest fragments and gallery forests, which local people (farmers, farm workers and hunters with demonstrable expertise in the local fauna; Davis & Wagner, Reference Davis and Wagner2003) indicated as having resident titi monkeys. We conducted 124 interviews using formal questionnaires: 37 where the species was eventually recorded and 87 in areas where it was not found. We interviewed owners and employees of each farm we visited to obtain information on the size of the forest remnants, the presence of areas protected by Brazilian law (e.g. Legal Reserves), land use (agriculture, cattle ranching, fruit crops or mining), and the extent and impact of hunting and other threats affecting the species. Surveys were carried out in the early morning when titi monkeys are more active and often call. We played recordings of calls of the closely related Callicebus personatus from Emmons et al. (Reference Emmons, Whitney and Ross1997) to elicit responses by titi monkeys (37% of the new locality records resulted from vocal responses to the playback recording).
The vegetation was classified based on physiognomy and the presence of typical plant species, following Andrade-Lima (Reference Andrade-Lima1981): Dense Arboreal Caatinga (closed canopy, 20 m in height), Open Arboreal Caatinga (sparser forest with a broken canopy), Dense Shrubby Caatinga (Carrasco in western Bahia; cacti, shrubs, sparse trees, predominantly Euphorbiaceae); Sparse Shrubby Caatinga (cacti, shrubs), and Highland Coastal Rainforest (also called Agreste; deciduous pre-montane and montane, palm trees, arboreal cacti, epiphytic bromeliads, transitional between the caatinga and the rainforest sensu stricto), and Gallery Forest.
We estimated the extent of occurrence of C. barbarabrownae as the area of the polygon with angles ≤ 180º that encompasses all records. Area of occupancy was estimated by overlaying a grid with cells of area 0.01 cm2 (equivalent to an area of 0.25 km2 on a map scale of 1 : 5,000,000). Titi monkeys live in family groups of 2–4 individuals (adult pairs and up to two offspring). We estimated the size of the C. barbarabrownae population by the number of groups we located, considering each individual sighting or vocal contact as representing a social group of four.
We obtained 37 new locality records for C. barbarabrownae, all in Bahia (Table 1, Fig. 1). The northern limit of the species’ distribution was identified as the Serra do Minuim (Table 1, Fig. 1: locality 10), the southern limit the Serra do Sincorá Lamarão (locality 12), the eastern limit the municipality of Coronel João Sá (locality 8) on the border of the states of Bahia and Sergipe, and the western limit the municipality of Gentio do Ouro, 107 km from the Rio São Francisco (locality 36). Extent of occurrence was 291,438 km2 over altitudes of 241–908 m (Table 1). Area of occupancy was 2,636 km2. The population size was estimated to be 260 based on records of 65 social groups (51 individuals sighted and 14 vocal contacts). The species was not found in any protected area.
Agriculture was the major economic activity in 56% of the farms inhabited by titi monkeys, followed by cattle ranching (28%), and diversified land use (combinations of coffee plantations, horticulture, sheep farming, and fruit growing, e.g. watermelon and mango). Agriculture, especially of beans (24%), maize (22%) and manioc (4%), was the major economic activity in 50% of the farms where titi monkeys were not found, whereas cattle ranching accounted for 15% and diversified land use for the remaining 35%.
C. barbarabrownae is endemic to the caatinga but occupies < 30% of this biome. In the north it does not reach the Rio São Francisco, as had been proposed by Hershkovitz (Reference Hershkovitz1990). Canudos and Minuim, < 100 km south of the Rio São Francisco, are the current northern limits of its range. This study confirmed Hershkovitz’s (Reference Hershkovitz1990) supposition that the relief (or phytogeographical changes related to it) of the western Chapada Diamantina is a barrier for the species.
A lack of sightings of C. barbarabrownae in Dense Shrubby Caatinga and Gallery Forest, both seemingly adequate habitats, was significant because both provide arboreal corridors between the many isolated patches of Dense Arboreal Caatinga and Highland Coastal Rainforest where most of the remaining populations were found. The ubiquitous loss of Gallery Forest compromises habitat connectivity for arboreal species in the caatinga. Different forms of land use also affect the persistence of C. barbarabrownae in habitat patches. Although the prevalence of agriculture was not related to the likelihood of occurrence of titi monkeys, cattle-ranching was. This is possibly because cattle ranches have larger forest reserves (the stipulated Legal Reserve comprising a minimum of 20% of a landholding; Brazilian Federal Law 4771/1965) than crop farms, and a larger number of cattle ranches maintained titi monkeys than those which did not.
The urbanization of rural areas has probably contributed to the local extinction of the species from many areas. Feira de Santana and Serrinha are examples of municipalities that have experienced significant human population growth and where titi monkeys have now disappeared. Extirpation near human settlements is due not only to hunting but also to the fragmentation, degradation and disturbance of vegetation, and exposure to dangers such as dogs and electrocution on power lines, and road kill (Printes, Reference Printes1999; Lokschin et al., Reference Lokschin, Printes, Buss and Cabral2007).
Our estimate of the total population size for C. barbarabrownae is a minimum and an underestimate because the species is cryptic and shy. Some vocal contacts may have involved more than one group. However, it is unlikely that the species reaches a minimum functional viable population size because of the wide dispersion of its small and isolated subpopulations in tiny forest patches over 291,438 km2. Less than 4% of the caatinga is officially protected (TNC do Brasil & Associação Caatinga, 2004) and blond titi monkeys were not found in any protected area. The isolation of the known remnant populations poses significant challenges to dispersal. Our baseline data on the distribution and size of these populations will help in designing future surveys at finer spatial scales, an urgent task for developing effective landscape conservation, habitat restoration and management strategies for C. barbarabrownae.
The assessment of C. barbarabrownae as Critically Endangered (Veiga et al., Reference Veiga, Printes, Rylands, Kierulff, de Oliveira and Mendes2008) was supported by our findings. Six years on, we have no indication that the status of the species has changed for the better or for the worse. A potential threat in the coming years is the transposition of the Rio São Francisco, a mega-project already underway to provide water to semiarid areas of four Brazilian states (Suassana, Reference Suassuna2010). Direct or indirect impacts could result from urban and transport infrastructure, agricultural development and increased human populations, although they will be concentrated to the north of the river; i.e. north of the species’ range. The creation of protected areas, measures to maintain and augment their habitats through reforestation, and further research on ecology and behaviour are needed to protect the blond titi monkey, one of the few mammals endemic to the caatinga and its threatened forests.
Acknowledgements
We thank the Primate Action Fund, Critical Ecosystem Partnership Fund, Fundação Biodiversitas, Instituto de Estudos Sócio-Ambientais do Sul da Bahia, Coordenação de Aperfeiçoamento do Ensino Superior, Centro de Proteção de Primatas Brasileiros, and the Núcleo de Extensão Macacos Urbanos for their support, and André C. Alonso, André Hirsch, Antônio Estrela, Ayr Müller Gonçalves, Carlos E. Guidorizzi, Cassiano Gatto, Elena Charlotte Landau, Gabriel dos Santos Rodrigues, Jader Marinho-Filho, Jaques Pinto Rangel, Jocélia Grazia, Leandro Jerusalinsky, Leonardo Oliveira, Luisa Lokschin, Marcos de Souza Fialho, Marcelo Sousa, Marcelo Xavier, Maria Cecília Kierulff, Raquel Moura, Roberto W. Groehs and Waldney Martins.
Biographical sketches
Rodrigo Cambará Printes is an ecologist interested in primate conservation, reserve management and environmental policy development. He has conducted research on brown howler monkeys, northern muriquis, and blond titi monkeys. Anthony Rylands is a zoologist with wide interests in primate conservation, systematics, and ecology. Júlio César Bicca-marques is an anthropologist whose research focuses on primate ecology, behaviour, cognition and conservation biology.





