Introduction
Veterinary use of diclofenac, a non-steroidal anti-inflammatory drug (NSAID), is the main factor responsible for the declines of species of Gyps vultures in South Asia. Studies have established that diclofenac is toxic to vultures (Oaks et al., Reference Oaks, Gilbert, Virani, Watson, Meteyer and Rideout2004; Swan et al., Reference Swan, Cuthbert, Quevedo, Green, Pain and Bartels2006a) and is widespread in cattle carcasses across India (Taggart et al., Reference Taggart, Senacha, Green, Jhala, Raghavan and Rahmani2007) at sufficient concentrations to be the principal cause of the declines (Green et al., Reference Green, Taggart, Das, Pain, Sashikumar, Cunningham and Cuthbert2006, Reference Green, Taggart, Senacha, Pain, Jhala and Cuthbert2007). As a consequence, the manufacture and importation of veterinary diclofenac was banned in India in May 2006, with bans in Nepal and Pakistan in the same year. Further legal measures in India, in August 2008, placed additional restrictions prohibiting the manufacture, sale, distribution and veterinary use of diclofenac. Prior to the 2006 ban, testing of an alternative NSAID, meloxicam, indicated that this drug is of low toxicity to Gyps vultures and unlikely to cause death at plausible exposure levels. This drug is widely used as an effective veterinary medicine elsewhere (Cuthbert et al., Reference Cuthbert, Parry-Jones, Green and Pain2006; Swan et al., Reference Swan, Naidoo, Cuthbert, Green, Pain and Swarup2006b; Swarup et al., Reference Swarup, Patra, Prakash, Cuthbert, Das and Avari2007) and consequently meloxicam was proposed as a viable alternative to replace the use of diclofenac in South Asia. To date, information on the prevalence of diclofenac and meloxicam in the environment has been obtained from sampling a large number of carcasses of domestic ungulates available to vultures at many carcass dumps across India (Senacha et al., Reference Senacha, Taggart, Rahmani, Jhala, Cuthbert, Pain and Green2008) and analysing liver samples for the presence of these drugs (Taggart et al., Reference Taggart, Senacha, Green, Cuthbert, Jhala and Rahmani2009). These surveys indicate that, prior to and immediately after the 2006 ban, diclofenac residues were detectable in 10–11% of carcasses across northern India (Taggart et al., Reference Taggart, Senacha, Green, Jhala, Raghavan and Rahmani2007, Reference Taggart, Senacha, Green, Cuthbert, Jhala and Rahmani2009). As carcasses still comprise the principal food source of vultures in India this monitoring is one of the most direct means of measuring their exposure to NSAIDs and monitoring the 2006 diclofenac ban.
A complementary approach to surveys of ungulate carcasses is to survey veterinary pharmacies to assess the availability of diclofenac and other NSAIDs for purchase. Here we summarize findings from pharmacy surveys across India during late 2007 to mid 2010: 1.5–4 years after the diclofenac ban was imposed. Our objectives were to determine the range of veterinary NSAIDs on sale, to assess the availability of diclofenac and meloxicam after the 2006 diclofenac ban, and to make recommendations to strengthen the effectiveness of conservation actions to protect Asia’s vultures.
Methods
Veterinary pharmacies were visited in 11 states between November 2007 and June 2010. All pharmacies visited were in cities and towns and were likely to be legally registered and managed by qualified pharmacists. Information on the type of compound (defined by the active NSAIDs within the compound) and brands (a company’s individual version of a compound) of NSAIDs available for purchase were obtained by visiting > 250 pharmacies, with data recorded from 176 that stocked at least one form of NSAID compound. Field biologists, veterinarians and trained volunteers, all Indian nationals (generally from the same state as the location of the pharmacies) visited the pharmacies and asked to buy NSAIDs for treating livestock. No attempt was made to pretend that surveyors were farmers or livestock owners or to steer pharmacists into offering any particular type of NSAID for sale, with the exception of surveys in Uttarakhand state where a local man and livestock owner requested treatment for a sick cow or buffalo and indicated they had used diclofenac previously (Mahseer Conservancy, 2009). Standard forms were completed for all pharmacies visited, recording the date of the visit, pharmacy name and location; when possible a sample of each NSAID was purchased at each pharmacy. Subsequently, details on the type of NSAID, number of brands, if the drug was in an injectable or bolus (oral tablet) form, and manufacturing date and price were recorded. In Kerala, Tamil Nadu and Karnataka (all in south India) and in Jharkhand, the shopkeepers were questioned (after NSAID purchase had been attempted) on whether they were aware of the ban on diclofenac and the role of diclofenac in the decline of vultures, and whether diclofenac for human use was available for veterinary use. The number of pharmacies where relevant information could be collected varied among states and consequently data from some adjacent states were pooled to provide sufficiently large samples for meaningful comparison. Areas were grouped as south (comprising 26 pharmacies in Andhra Pradesh (n = 6), Karnataka (3), Kerala (11) and Tamil Nadu (6)), central (31, in Madhya Pradesh (6) and Maharashtra (25)), west (57 in Rajasthan (22) and Gujarat (35)), and north India (27, from Uttarakhand (21) and Jammu & Kashmir (6)). Data for Jharkhand state (35) were presented individually. As the majority of pharmacies only stocked one brand of any particular NSAID, nationwide comparisons and area comparisons are based on the presence/absence of the type of NSAIDs held in pharmacies, rather than on the number of brands per pharmacy.
Results
Across all states 83 bolus brands and 80 injectable brands of NSAIDs were recorded as offered for sale to treat livestock. Meloxicam, diclofenac, nimesulide and analgin were the NSAIDs with the greatest number of manufacturer’s brands (Table 1). In total 12 types of NSAID were found on sale: aceclofenac, analgin (also known as metamizole), diclofenac, flunixin meglumine, ibuprofen, ketoprofen, mefenamic acid, meloxicam, nimelsulide, paracetamol (also known as acetaminophen), phenyl butazone and piroxicam. NSAIDs on sale were frequently found to contain more than one active ingredient, with paracetamol included as a secondary ingredient in 55% of bolus formulations and 20% of injectable formulations. Paracetamol was most frequently combined with bolus forms of nimelsulide, injectable and bolus forms of meloxicam, and bolus forms of diclofenac (Table 1). Forty-two brands of diclofenac alone or combined diclofenac and paracetamol were found in the survey, including 16 brands of bolus and 26 injectable brands. All 26 of the injectable brands of diclofenac that were purchased were manufactured for human use but sold for veterinary treatment. Nine brands of diclofenac bolus that were purchased were manufactured after the 2006 ban, all for veterinary use.
Table 1 Number of brands of bolus and injectable formulations of NSAIDs and the combined total number from a survey of 11 Indian states during 2007–2010. Numbers in parentheses indicate the number of brands in which paracetamol was a secondary active ingredient.
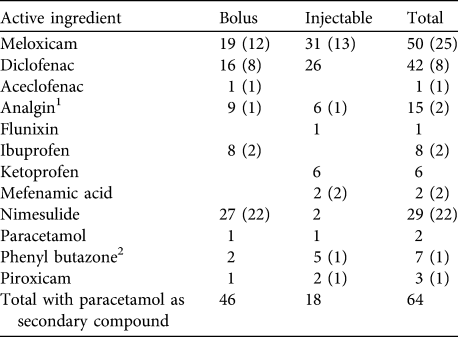
1 Two bolus and three injectable brands of analgin were formulated with phenyl butazone
2 One brand of injectable phenyl butazone was formulated with sodium salicylate (aspirin)
For pharmacies that sold NSAIDs the median number of brands per compound held by each was 1, with shops only holding more than one brand of meloxicam and meloxicam + paracetamol bolus brands (mean 1.1 ± SE 0.04 brands, range 1–2) and meloxicam and meloxicam + paracetamol injectable brands (mean 1.2 ± SE 0.06 brands, range 1–4). On average pharmacies sold 4.0 ± SE 0.2 different NSAID compounds and 4.3 ± SE 0.2 brands. Combining information from all 11 states, meloxicam was the most commonly encountered NSAID, present in 70% of all 176 pharmacies that sold any type of NSAID (Fig. 1). Of the pharmacies selling meloxicam 31% sold both injectable and bolus forms of meloxicam, with 31% selling only injectable formulations and 7% only bolus formulations. Nimelsulide (48%) and analgin (47%) were the next most frequently encountered NSAIDs. Diclofenac and ketoprofen (both toxic to vultures) were recorded in 36 and 29% of pharmacies surveyed, respectively. Other NSAIDs were only recorded in < 10% of pharmacies surveyed (Fig. 1), with aceclofenac, flunixin and paracetamol recorded from only one or two pharmacies.
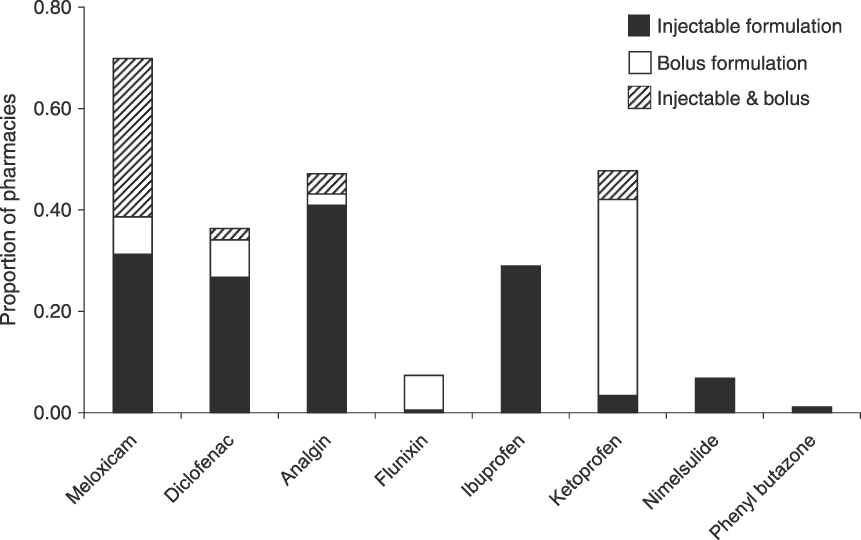
Fig. 1 Prevalence of eight NSAIDs across all 11 states surveyed, indicating the proportion of pharmacies holding only injectable formulations, only bolus formulations and both. In addition the NSAIDs flunixin, mefenamic acid and paracetamol (as a single compound) were also recorded but from a very small proportion of pharmacies (1–2).
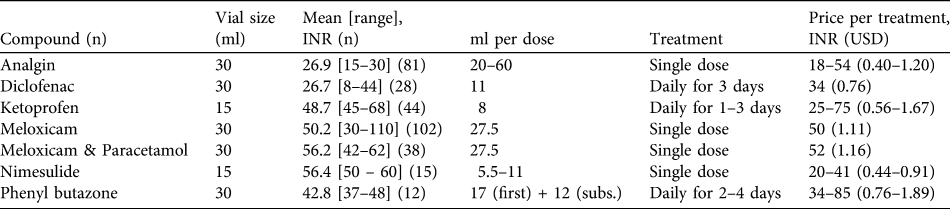
Injectable brands were available in a range of sizes, with 30 ml vials being most frequently sold (82.8% of 460 vials purchased) followed by 15-ml vials (12.8%), and with vials of 3, 5, 10, 25, 50 and 100 ml also available but comparatively rare (0.2–1.5%). The average price per 30- or 15-ml vial were similar for ketoprofen, meloxicam, meloxicam + paracetamol, nimelsulide and phenyl butazone at c. INR 40–60 (USD 0.89–1.33). However both diclofenac and analgin were cheaper (Table 2). When the treatment costs of different NSAIDs are compared (for dosing a 275 kg cow Bos indicus) the price differential between meloxicam and diclofenac is reduced but diclofenac remains a cheaper treatment (Table 2).
Table 2 Vial size, mean and range of price, ml of compound per dose, treatment length, and price per treatment for seven injectable compounds purchased from pharmacies (see text for details). Dosages are based on a medium mass of 275 kg for Indian cattle Bos indicus (adult mass 250–300 kg; Kumar Ghosh, Reference Kumar Ghosh1998) and dose information published by CinVEX (Reference Radha2001), Merck Veterinary Manual (2010) and Agrawal & Gupta (Reference Agrawal and Gupta2010).
Comparison between areas indicates differences in the prevalence of NSAIDs, in particular the availability of diclofenac and ketoprofen (Fig. 2). Diclofenac was most frequently encountered in central and western India where it was recorded in 44–45% of pharmacies visited. In contrast, diclofenac was only found in 4 of 35 pharmacies (11%) in Jharkhand. The prevalence of ketoprofen also varied widely, ranging from 4% in north India to 46% in Jharkhand state. Meloxicam was found at a high prevalence in all areas (65–89% of pharmacies) other than in north India where it was only found in 22% of pharmacies. Information on the shopkeepers’ awareness of the diclofenac ban in the southern states of Kerala, Tamil Nadu and Karnataka indicated a considerable lack of awareness of the ban and the role of diclofenac in vulture declines, with only 4 of 18 pharmacies (22%) aware of the ban. In contrast, 25 of 35 pharmacies (71%) were aware of the ban on diclofenac in Jharkhand. Ten pharmacists (in Uttarakhand, Kerala and Gujarat) offering brands of diclofenac for human use also advised that higher dose rates be used for veterinary treatment.
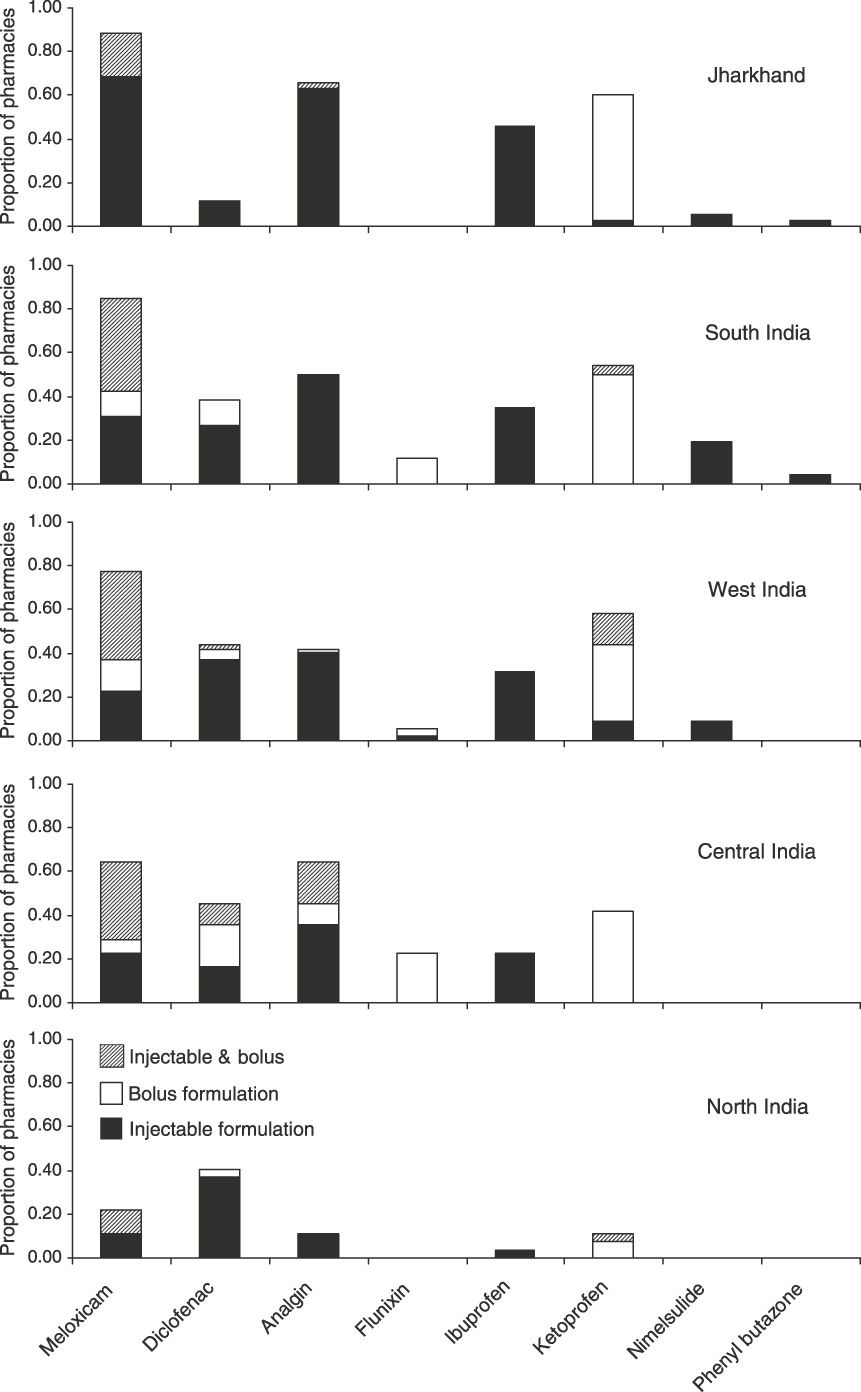
Fig. 2 Prevalence of eight NSAIDs by area (see text for details) indicating the proportion of pharmacies holding only injectable formulations, only bolus formulations and both. Areas are ordered from the least (top) to highest (bottom) diclofenac prevalence.
Discussion
Availability of diclofenac and other NSAIDs
A major concern arising from this study is the widespread availability of diclofenac for sale for veterinary use in India after the June 2006 diclofenac ban. Our first surveys were from September 2007 to March 2008 and in the four states surveyed (Rajasthan, Maharashtra, Madhya Pradesh and Gujarat) we recorded diclofenac on sale for veterinary use in 43% of pharmacies. The most recent surveys, from August 2009 to June 2010 in Rajasthan, Maharashtra and Uttarakhand states, found diclofenac for sale for veterinary use in 47% of pharmacies surveyed, indicating that despite national legislation to ban the veterinary use of diclofenac the drug remains widely available for sale. While national legislation has been effective at removing most veterinary formulations of diclofenac from the country, the ban on diclofenac is being circumvented through the sale of forms of the drug for human use. All the injectable formulations of diclofenac offered for sale were for human use and therefore the manufacture was legal. However, they were being offered for sale for veterinary use, which is illegal. Bolus (tablet) formulations were made for veterinary use and are therefore illegal, with nine bolus brands found manufactured after the 2006 ban.
While illegal selling of diclofenac is of major concern, there is no evidence that any compounds were mislabelled by manufacturers, as would occur if manufacturers were trying to sell old stocks of diclofenac as another drug. All NSAIDs purchased were manufactured in India, and labelled in English with appropriate detail on the concentration of active ingredients and date of manufacture. An analysis of 39 injectable brands of seven different NSAIDs using a validated methodology (Taggart et al., Reference Taggart, Senacha, Green, Cuthbert, Jhala and Rahmani2009) found that all contained the active ingredients specified on the label (Royal Society for the Protection of Birds, unpubl. data).
As well as the continued high availability of diclofenac, the large range of NSAIDs for sale in India is of additional concern as, with the exception of meloxicam, diclofenac and ketoprofen, little is known of the toxicity or safety of these other NSAIDs. Ketoprofen, which is toxic to vultures and found in livestock carcasses in India (Naidoo et al., 2009; Taggart et al., Reference Taggart, Senacha, Green, Cuthbert, Jhala and Rahmani2009), was recorded from c. 33% of pharmacies, suggesting that it is becoming widely used. Six brands of ketoprofen compounds were found on sale (Ketop, Neoprofen, Vetoprofen, Ketolon, Butagesic-K and Vetprofen), all injectable formulations. Aceclofenac was found in two pharmacies in Jharkhand, with bolus forms of this NSAID combined with paracetamol. While the manufacture and sale of aceclofenac is legal, this compound has a similar chemical structure to diclofenac and in rats, monkeys and humans is metabolised to diclofenac and 4’-hydroxydiclofenac (Bort et al., Reference Bort, Ponsoda, Carrasco, Gómez-Lechón and Castell1996; Yamazaki et al., Reference Yamazaki, Kawai, Matsumoto, Matsuzaki, Hashimoto and Yokokura1999). Given this metabolic pathway, the use of aceclofenac in cattle could potentially have the same toxic consequences to vultures as administering diclofenac, and further research is required to establish if this is the case as this drug is apparently gaining in popularity in India (P. Sharma, pers. comm.). Flunixin, which may be toxic to scavenging birds (Cuthbert et al., Reference Cuthbert, Parry-Jones, Green and Pain2006), was recorded from one pharmacy in Rajasthan. Nothing is known of the safety or toxicity of analgin and nimelsulide despite the widespread availability of these NSAIDs in pharmacies. The safety or toxicity of paracetamol (acetaminophen) to vultures and other scavenging birds is unknown but this compound is frequently used in combination with other drugs, including both injectable and bolus formulations of meloxicam.
Availability of meloxicam
Conservationists in India have promoted the sale and use of meloxicam as it is the only NSAID that safety testing has established is of low toxicity to vultures as well as being an effective NSAID for treating livestock (Cuthbert et al., Reference Cuthbert, Parry-Jones, Green and Pain2006; Swan et al., Reference Swan, Naidoo, Cuthbert, Green, Pain and Swarup2006b; Swarup et al., Reference Swarup, Patra, Prakash, Cuthbert, Das and Avari2007). Among the wide range of NSAIDs sold by pharmacies in India more brands of meloxicam were found for sale than any other compound, with 19 bolus and 31 injectable manufacturing brands purchased. In 2006, when meloxicam was announced as a safe alternative to diclofenac (MoEF, 2006; Swan et al., Reference Swan, Naidoo, Cuthbert, Green, Pain and Swarup2006b), only one or two companies were manufacturing veterinary meloxicam in India. The number of brands now available indicates that a large number of pharmaceutical companies are manufacturing and marketing this drug since its recommendation as a replacement for diclofenac, with at least 22 companies known to be manufacturing meloxicam as of November 2010 (Bombay Natural History Society, unpubl. data). Based on its prevalence in pharmacies, meloxicam may be the most commonly used veterinary NSAID for treating livestock, representing a significant shift in use of this compound. The widespread availability of meloxicam is of further importance, as veterinary medicines are not just bought and used by trained veterinarians but are also administered by farmers, and para-vets and quacks (Indian terms for veterinary technicians who have received some training and for unqualified veterinary practitioners, respectively). Reaching and educating veterinary professionals on the role of diclofenac is relatively easy in comparison to targeting the more numerous untrained practitioners and farmers who work in the rural areas of India, and the widespread availability of meloxicam will directly influence what NSAIDs can be purchased and administered by this group.
Conservation recommendations
The increasingly widespread availability of meloxicam in pharmacies in India is encouraging. However, the continued sale of diclofenac for veterinary use and the wide range of untested drugs that are also available for sale are of concern. Although the legal actions to outlaw veterinary use of diclofenac have been successful in removing veterinary formulations of this drug from the market, the law is being circumvented through the sale of formulations of diclofenac for human use that are sold for veterinary use. Two potential actions that could help eliminate the misuse of such formulations are for states in India to take legal action against companies or individuals, and to alter and restrict the vial size of formulations of diclofenac for human use to make these less practical for veterinary use. If such measures fail to be effective in preventing veterinary use then further restrictions or even a complete ban on the sale of diclofenac for human use may also need to be considered.
To date, prosecutions relating to the sale or manufacture of diclofenac for veterinary use have been rare, with only Bihar state taking the step of filing a prosecution against a company, Raathi Laboratories (Hindusthan) Private limited, in November 2009 (Vulture Rescue, 2010). Such steps could potentially be taken against a number of pharmacies that are illegally selling diclofenac intended for human use for veterinary purposes, as well as against any pharmaceutical manufacturers that are still illegally making bolus forms of diclofenac. Such actions would provide a strong deterrent as well as increased awareness of the diclofenac ban amongst pharmaceutical users, sellers and manufacturers.
Legislation to restrict the size of vials of diclofenac for human use could also be effective in reducing misuse of diclofenac, as this will increase the costs of using these formulations for veterinary treatment. Injectable diclofenac in 3-ml vials cost INR 2.1–4.1 per ml of compound (USD 0.05–0.09 per ml) versus an average price of INR 0.9 per ml for a 30-ml vial (USD 0.02 per ml). Veterinarians and farmers typically inject a whole 30-ml vial of diclofenac when treating cattle (P. Averi, pers. comm.) and consequently the cost of treating an animal with multiple 3-ml vials would be INR 62–123 (USD 1.38–2.73), versus an average cost of INR 27 (USD 0.59) for a single 30-ml vial. This change in vial size would make diclofenac treatment more expensive than meloxicam treatment (Table 2), a factor we consider of critical importance for the elimination of diclofenac from veterinary use.
In addition, we also urge that pharmaceutical companies take proactive responsibility for testing the safety or toxicity of the drugs they are manufacturing. Little is known of the potential toxicity or safety of most of the NSAIDs on sale in India, with only the safety of meloxicam and toxicity of diclofenac and ketoprofen confirmed. Establishing the safety or toxicity of the other NSAIDs on sale for veterinary use in South Asia is a priority and should be the responsibility of the pharmaceutical companies manufacturing these drugs rather than the responsibility of conservation organizations that have so far taken the lead in testing. Given the small number of brands and companies manufacturing ketoprofen, targeting these companies with information about this drug’s toxicity could be undertaken relatively easily. Action at a national level should also be taken to ban the manufacture and use of ketoprofen for veterinary purposes.
In conclusion, this study has demonstrated the wide range of NSAIDs available for veterinary use in India and the continued availability of diclofenac in pharmacies. We recommend that continued monitoring, for which the results of this study are a baseline, should be undertaken in the same areas to evaluate the future availability of NSAIDs across India. To prevent the misuse of these compounds, establishing the safety or toxicity of other NSAIDs is a priority, along with action against pharmaceutical companies and pharmacies that are breaking the law, and legal restrictions on the size of vials of diclofenac manufactured for human use.
Acknowledgements
Funding for this study was made available from the Royal Society for the Protection of Birds and from the UK Government’s Darwin Initiative. We are grateful to Chris Bowden, Rhys Green and two anonymous reviewers for useful comments.
Biographical sketches
Richard Cuthbert is involved in research to halt the decline of Asia's threatened vultures, in research and conservation projects investigating the impact of invasive species in the UK overseas territories, and in the conservation of albatrosses, petrels and penguins. Vibhu Prakash directs the Bombay Natural History Society's vulture programme and vulture conservation breeding centres. He was responsible for discovering the catastrophic declines of vultures across India and has a lifelong passion for raptors. Ruchi Dave, Souma Chakraborty, Sashi Kumar and Sachin Ranade all work with the Bombay Natural History Society and undertake research, advocacy and conservation action to conserve India's vultures. Satya Prakash is the director of the Neo Human Foundation, which works for an integrated approach to environmental conservation. As well as being involved in vulture conservation he has a keen interest in the ecology and conservation of snakes in Jharkhand state.






