1 Introduction
Mineral grains are the building blocks of clastic sediments. The mixture of minerals together with organic matter influences the pore water composition, the soil's pedological evolution and fertility, the fate of pollutants in the subsurface, the porosity and permeability as geohydrological properties and the geomechanical strength of the subsurface. More specifically, mineralogical composition plays an important role in sediment reactivity (Van Gaans et al., Reference Van Gaans, Griffioen, Mol and Klaver2011), greatly influencing surface complexation and ion exchange of cations and anions, sorption of organic substances, denitrification and other redox reactions, as well as the weathering processes. Insight into this reactivity is needed for environmental management issues such as drainage-induced oxidation, aquifer storage and recovery, natural attenuation of pollutants, leaching of nutrients from farmland, reactive transport and secondary mobilisation of trace metals, and transfer of pollutants or nutrients from aquatic sediments to the water column or vice versa. Additionally, the reactivity of sediment influences the pH and redox state of the subsurface and hence the stability of the subsurface, man-made constructions and buried materials (examples being the corrosion of steel constructions and the stability of archaeological heritage).
The mineralogy of sediments is also used to stratigraphically classify sediments and to characterise their provenance. Heavy minerals (i.e. having a specific density exceeding 2.85 kg/dm3; Boggs, Reference Boggs2009) are traditionally used for this purpose, but this is labour-intensive and is nowadays rarely applied in the Netherlands. The clay mineralogy may also be used as a palaeogeographical tool to characterise past climatological conditions and the provenance of sediments (Tebbens et al., Reference Tebbens, Veldkamp and Kroonenberg1998; Thiry, Reference Thiry2000; Zeelmaekers, Reference Zeelmaekers2011). The nature of quartz, mica and feldspar grains also assists in the visual recognition of different kinds of sediments: the presence or absence of multicoloured quartz and of mica are important criteria when visually classifying Dutch fluvial and other sediments stratigraphically (De Mulder et al., Reference De Mulder, Geluk, Ritsema, Westerhoff and Wong2003). Other criteria often used are the presence of glauconite, shells or, more generally, Ca carbonate (using the effervescence test).
More abstractly, sediments and suspended matter are both important in the biogeochemical cycling of the elements and the functioning of ecosystems. For example, the source/sink function of dissolved substances and the food function of benthic organisms are controlled by the reactivity of sediments (including the state of particulate organic matter) and thus by their mineralogy. Additionally, the factors determining the erosion–transport–sedimentation behaviour and related flocculation/deflocculation behaviour include the physico-chemical properties of the minerals involved.
From the foregoing it is clear that insight into the mineralogical composition of sediments is of general relevance. This being the case, it is surprising that there has been no overview of the mineralogical composition of sediments in the Netherlands. A wealth of information is available on the geology of the Netherlands, and several books present geological overviews of the country (De Mulder et al., Reference De Mulder, Geluk, Ritsema, Westerhoff and Wong2003; Wong et al., Reference Wong, Batjes and De Jager2007 are the most recent and extensive ones). However, these kinds of texts address the mineralogy in a qualitative and restricted way, focusing on distinguishing and grouping heavy minerals for stratigraphical reasons. This paper therefore aims to provide an overview of the mineralogy and organic geochemistry of sediments as well as suspended matter as found in the Netherlands (and some parts of Belgium), based on an extensive review of the scientific literature of more than a century. We address suspended matter, recent sediments of the surface water systems and Cenozoic sediments because they form a geoscientific continuum: recent marine and fluvial sediments as well as suspended matter are the present-day analogues of the geological sediments. Most attention is paid to the following groups of minerals, with an indication of their relevance: heavy minerals as indicators of provenance etc., clay minerals for their contribution to the sorption capacity and impact on geohydrological and geomechanical properties, feldspars as major, weatherable Al silicates, Ca carbonates as reactive, pH-buffering minerals, reactive Fe minerals (oxides, carbonates, sulphides, glauconite) for their overall reactive behaviour, and solid organic matter for its redox-controlling and sorptive capacities. The review has been limited to the Cenozoic sediments because there is little literature on older sediments for which integration is less needed (an exception being the Rotliegend Sandstone, which is the most important natural gas reservoir rock in the Netherlands and has therefore been intensively studied; Gaupp & Okkerman, Reference Gaupp and Okkerman2011). The sediments are predominantly clastic; organic or chemical sediments play a minor role in the Cenozoic records of the Netherlands.
Fig. 1 presents a map of the Netherlands with all geographical names used in this paper. Section 2 presents a brief overview of the geology and geography of the Netherlands. The review method is described in section 3, together with several methodological analytical approaches that have been applied by researchers. In the Netherlands, the mineralogy of fluvial and marine environments has been studied much more intensively than that of glacial, aeolian, lacustrine and peat environments, and so separate sections (4 and 5) are devoted to them. When possible and relevant, the amounts, provenance, relationship with grain size, early diagenesis and palaeohydrological evolution are described per type of mineral. The findings for the other sedimentological environments are fragmented; they are described in section 6. The general discussion presented in section 7 evaluates and integrates several of the findings.

Fig. 1. The Netherlands, showing the topography and the locations of the geographical names referred to in this paper.
2 Geoscientific characteristics of the Netherlands
2.1 Geological history
The Netherlands lies on part of the lowlands bordering the southern part of the North Sea. Geologically, the area has evolved largely from sedimentary processes in a deltaic setting at the lower end of major river systems draining into the southern North Sea (Fig. 2). Tectonically, the Netherlands is part of the subsiding North Sea Basin. It is bounded in the southeast by the Palaeozoic rocks of the Ardennes and Eifel, and continues to the east in the plains of North Germany and Poland. The main tectonic structure of the subsurface is situated in the southeast of the country, where major fault zones trending southeast to northwest border the subsiding Roer Valley Graben and the adjacent Peel Block. The latter is strongly dissected by a complex pattern of faults (Van Balen et al., Reference Van Balen, Houtgast and Cloetingh2005).

Fig. 2. Geological settings of the Netherlands during the Quaternary (upper left) and its main, shallow tectonic structures.
During most of the Cenozoic (the last 65 million year of the Earth's history), the area was under shallow seas in which fine-grained sediment was deposited. Generally these shallow marine deposits show a trend of decreasing mean grain size towards the centre of the North Sea Basin, while at the margins fine- to medium-grained sand prevails. These sediments may have a characteristic green colour, which results from their glauconite content.
Local and regional uplift in the surrounding areas from about 10 million years ago brought about fluvial systems draining to the southern North Sea Basin. Large quantities of sediment were supplied to the North Sea Basin by these rivers, forming huge delta systems that gradually prograded westwards and northwestwards. By the time of the Mid-Pliocene (c. 3 million years ago), the coastline roughly followed the southern and eastern borders of the present territory of the Netherlands. Thereafter the coastline gradually shifted northwest, and in the course of the Early Pleistocene (about 1.5 million years ago) fluvial sedimentation dominated the entire part of the country that is now onshore. The sediment was supplied by four main river systems: the Rhine, the Meuse, the rivers draining the central and northern part of Belgium, and the Eridanos fluvial system (a major drainage system originating in the Baltic area). The Rhine, Meuse and Belgian rivers dominated the sedimentation in the southern and central part of the Netherlands. The Eridanos supplied sediment to the northern part of the country.
As a result of long-term fluvial-deltaic processes in a subsiding basin, the upper 200 m of the subsurface of the Netherlands consists of complex interdigitated marine and deltaic deposits overlain by a series of stacked fluvial deposits. In the northern and central part of the country this complex pattern of stacked marine and fluvial deposits was interrupted at least twice by major advances of ice sheets during Elsterian and Saalian glacial phases in the upper part of the Pleistocene. For additional information on the geological history of the Netherlands see De Mulder et al. (Reference De Mulder, Geluk, Ritsema, Westerhoff and Wong2003), Wong et al. (Reference Wong, Batjes and De Jager2007), Weerts et al. (Reference Weerts, Westerhoff, Cleveringa, Bierkens, Veldkamp and Rijsdijk2005), Westerhoff (Reference Westerhoff2009) and Knox et al.(Reference Knox, Bosch, Rasmussen, Heilmann-Clausen, Hiss, de Lugt, Kasinski, King, Köthe, Słodkowska, Standke, Vandenberghe, Doornenbal and Stevenson2010).
2.2 Geography and hydrology
Geographically as well as geologically, the landscape of the Netherlands can be subdivided into two major domains. The coastal and alluvial lowlands form the rather flat and low-lying western and northern parts of the country (Fig. 1). This area is covered by a seaward thickening wedge of Holocene tidal and fluvial deposits (Fig. 3), which serve as a confined, semi-permeable cover on top of coarser-grained Pleistocene deposits. Generally, the elevation of the coastal plain is at or slightly above mean sea level, but in the reclaimed areas (polders) the ground level may be several metres below that level. Surface water is pumped out of the polders and groundwater recharge happens by infiltration of rain, seawater and surface water (lakes and also ditches or canals in those polders that have a relatively higher altitude). Recharge of the aquifers from relatively high-lying polders is 30–100 mm/y (NHV, 2004). Groundwater within 30 m depth is usually pH-neutral and anaerobic, and varies from fresh to saline (Griffioen et al., Reference Griffioen, Vermooten and Janssen2013). The conditions for weathering of the aquifer sediments are thus generally unfavourable. A complex of dunes and barriers lies along the entire Dutch coast, with a maximum height of 55 m above sea level. The fresh groundwater belt in the dunes is a major source of recharge for the adjacent low-lying polders and is nowadays used for aquifer storage and recovery of drinking water. Tidal estuaries typify the southwestern part of the coast, while extensive tidal flats and barrier islands characterise the northern coastline.

Fig. 3. Surface geology of the Netherlands.
Outside South Limburg, the rest of the country is often referred to as the ‘Pleistocene’ part of the country (Fig. 3). The terrain is smoothly undulating, with the surface mainly comprising sandy deposits formed during the Pleistocene Epoch and well-developed aquifers below. In the centre and east of the country, low hills formed as ice-pushed ridges some 150,000 years ago, when the northern half of the country was covered by a major ice sheet. The northern part of the country is an extensive flat area with basal till (‘keileem’) at or near the surface. The till area is regionally poorly drained and as a result, extensive peat formation occurred. However, most of the peat was excavated for fuel in the 19th and 20th centuries. Generally, groundwater is regionally discharged through a natural drainage system of small-scale rivers and brooks, although many groundwater abstractions are also present nowadays. A thin veneer of Holocene alluvial deposits may occur in the associated valley systems. Groundwater recharge is around 350 mm/y but lower under pine forest; the ice-pushed ridges act as major regional recharge areas when sandy. Shallow groundwater (<30 m depth) in the Pleistocene area is usually fresh, suboxic to anaerobic and acid to pH-neutral (Fest et al., Reference Fest, Temminghoff, Griffioen, Van der Grift and Van Riemsdijk2007; Griffioen et al., Reference Griffioen, Vermooten and Janssen2013). In the Pleistocene part of the Netherlands, leaching of the aquifers sediments is thus a feasible process at the geological time scale.
Mesozoic marls are outcropping in a small area of the Achterhoek in the eastern part of the country. A totally different landscape occurs in South Limburg, characterised by a more pronounced morphology of hills and incised valleys, with the highest elevation (322 m above sea level) at the Vaalserberg. Loess formed during the Middle and Late Pleistocene glacial periods forms here a veneer overlying Cretaceous and Paleogene and Neogene clastic marine and fluvial deposits. A sequence of Pliocene and Quaternary river terraces mainly consisting of coarse gravel marks the former course of the Meuse in the area. Groundwater is naturally recharged, typically fresh, frequently nitrate-rich and pH-neutral to slightly allkaline at shallow depth (Vermooten et al., Reference Vermooten, Maring, Van Vliet and Griffioen2007). The latter is due to the omnipresence of Ca carbonates.
The North Sea is an epicontinental sea with water depths of less than 30 m in the south to about 200 m in the north (Otto et al., Reference Otto, Zimmerman, Furnes, Mork, Saetre and Becker1990). Deeper water, up to 700 m, is restricted to Norwegian Trench and the Skagerrak far in the northeastern part of the North Sea. There is an anticlockwise rotating tidal and residual current system resulting in deposition of suspended matter in the estuaries, tidal flat areas near the Frisian Isles, the German Bight, the Skagerrak, Norwegian Channel and the Kattegat. The Frisian Front northwest of the Dutch Wadden island of Terschelling also acts as a temporary deposition area due to slow currents on average. The bottom of the shallow, southern part of the North Sea predominantly consists of sand stored in sand waves which is largely re-worked from fluvial deposits that were formed during extensive periods of low sea level during glacial phases. Most of the primary biological production takes place in the shallow unstratified southern part of the North Sea (Dauwe & Middelburg, Reference Dauwe and Middelburg1998).
2.3 Lithostratigraphy and geological formations
2.3.1 Lithostratigraphy
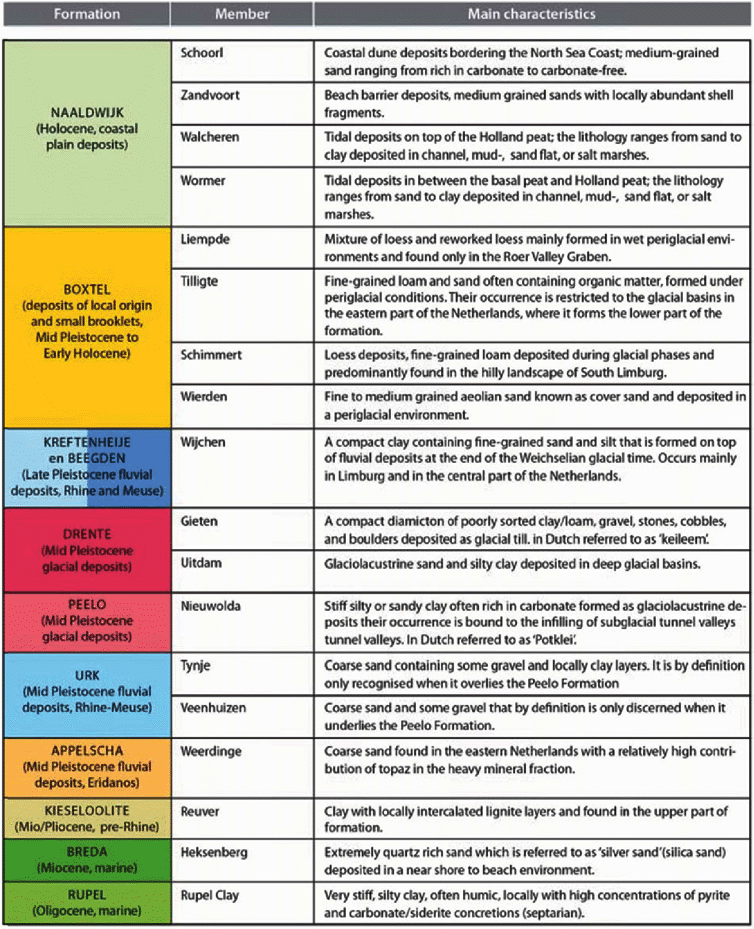
Coherent and mappable bodies of rock or sediment layers have been classified in the lithostratigraphical system of the Geological Survey of the Netherlands. In fact, lithostratigraphy is the classification of bodies of rock based on the observable lithological properties of strata and their relative stratigraphical positions (Salvador, Reference Salvador1994; Westerhoff & Weerts, Reference Westerhoff, Weerts and Elias2013). Lithostratigraphy serves to unravel the seemingly chaotic composition of the subsurface into geometrically (3D) defined units of more or less similar characteristics. It is a useful tool for showing the interrelationships of the discerned units. The central unit of the lithostratigraphical system applied here is the formation. Except for the lowermost marl deposits all Cenozoic formations are grouped in the so-called North Sea Group. Formations can be subdivided into members or beds (the lowest rank in the stratigraphical hierarchy). However, not all parts of a formation need to be subdivided into members or beds. In such cases the formation or a part of it is undifferentiated.In this review we deal with mainly surficial and shallow (up to a depth of few hundred metres) mostly unconsolidated Cenozoic deposits, which are found in most of the Netherlands. The lithostratigraphical subdivision of these Cenozoic deposits is summarised in Fig. 4. The various units are listed according to their geological age alongside the vertical. A striking modification with respect to a substantial part of the literature reviewed here is that the term Tertiary is no longer used. Instead, two periods, the older Paleogene and younger Neogene, now divide the Cenozoic time below the Quaternary. The lithostratigraphy of the Paleogene period follows the nomenclature published by Van Adrichem Boogaert and Kouwe (Reference Van Adrichem Boogaert and Kouwe1997), De Mulder et al. (Reference De Mulder, Geluk, Ritsema, Westerhoff and Wong2003) and Wong et al. (Reference Wong, Batjes and De Jager2007). The lithostratigraphy of the Neogene and Quaternary periods is in accordance with De Mulder et al. (Reference De Mulder, Geluk, Ritsema, Westerhoff and Wong2003) and Weerts et al. (Reference Weerts, Westerhoff, Cleveringa, Bierkens, Veldkamp and Rijsdijk2005). Both nomenclatures are found on DINOloket, the data and information site of the Geological Survey of the Netherlands (https://www.dinoloket.nl/nomenclator). Repeatedly, reference is made to members that are part of the geological formations. An overview of all members referred to is provided in Table 1.

Fig. 4. Lithostratigraphy of Cenozoic deposits in the Netherlands.
Table 1. Overview of the lithostratigraphical members mentioned in the subsequent sections of this manuscript, arranged according to the formations to which they belong, and some main lithological characteristics.

The literature reviewed and cited in this study repeatedly refers to the lithostratigraphical nomenclature published in the 1970s (Doppert et al., Reference Doppert, Ruegg, Van Staalduinen, Zagwijn, Zandstra, Zagwijn and Van Staalduinen1975), which was drastically revised in 2003 (Ebbing et al., Reference Ebbing, Weerts and Westerhoff2003; Weerts et al., Reference Weerts, Westerhoff, Cleveringa, Bierkens, Veldkamp and Rijsdijk2005; Westerhoff, Reference Westerhoff2009). The various Neogene and Quaternary lithostratigraphical units (Fig. 4) have now been categorised according to their genesis (i.e. marine, fluvial, glacial and deposits of mainly local or reworked origin) and the provenance of the sediment (i.e. Rhine, Meuse, Baltic area).
2.3.2 Paleogene deposits
Paleogene sediments are almost entirely marine. The lowermost, calcareous Paleogene sediments consist of soft fine- to coarse-grained marls and have been assigned to the Houthem Formation. The distribution is restricted to the southeast of North Brabant and Limburg. The formation forms part of the Cretaceous Chalk Group (Van Adrichem Boogaert & Kouwe, Reference Van Adrichem Boogaert and Kouwe1997).
All deposits younger than this formation form part of the North Sea Group, the basal part of which is formed by the Landen Formation. This formation consists of fine sand and clay, or a mixture of both, which was deposited in a shallow marine environment along the southern margins of the North Sea Basin.
The Dongen Formation is characterised by a sharp basal contact and consists of thick layers of stiff clay alternating with green glauconitic sand in the marginal areas. Towards the centre of the North Sea Basin the sand deposits grade into clay and clayey marls. The basal layer consists of a tuffaceous clay. The formation is of Eocene age. Regional uplift and inversion took place during the Pyrenean tectonic phase after deposition of the Dongen Formation and resulted in a hiatus in the upper part of the Eocene. Subsequently, the tectonic regime changed, and renewed subsidence took place during the Oligocene. It is noteworthy that the Roer Valley Graben has accommodated some 1200 m of sediment since the Late Oligocene, yet over the same period the adjacent Peel Block subsided by only some 200 m. Deposits of the Tongeren Formation occur only in South Limburg.
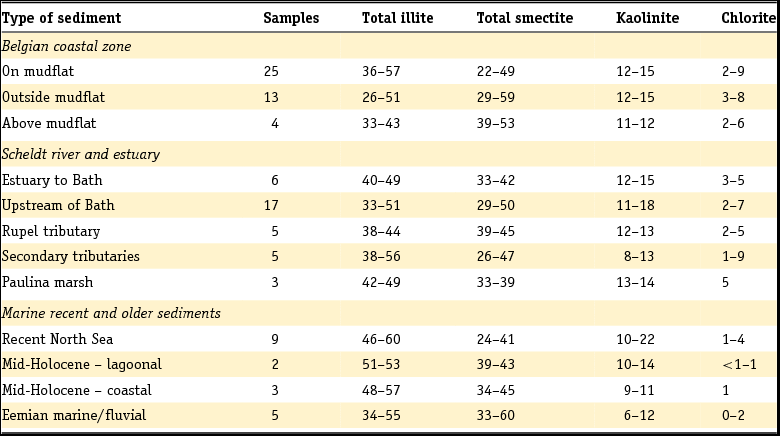
The Rupel Formation mainly consists of dark silt containing clay. The formation is subdivided into three main members. The lowermost one has been assigned as the Vessem Member and consists of fine-grained glauconitic sand. This member is up to 30 m thick and occurs mainly in the south of the Netherlands. It is overlain by the Rupel Clay Member, a dark brown to grey stiff clay with a relatively high silt content. The clay is up to 160 m thick and is absent in parts of South Limburg and in some areas along the eastern border of the country. In Zeeuws-Vlaanderen and parts of the Achterhoek, the upper part of the Rupel Clay Member occurs near to the surface. Although lithostratigraphically not identical, the Rupel Clay Member can be correlated with the well-known Boom Clay in Belgium, which has been studied intensively on its mineralogical composition. In this study we will refer to the deposit as Rupel Clay when we are discussing data and interpretations of the clay in the Netherlands. Conversely, we will speak of Boom Clay when dealing with information from Belgium.
The Veldhoven Formation is well developed in the Roer Valley Graben, where it is about 400 m thick. The distribution area is restricted to the Roer Vally Graben, the adjacent Peel Block and the southeastern part of the Zuiderzee Low. Tectonic activity caused regional uplift and inversion at the end of the Paleogene. Concurrently, the climate became cooler and sea level dropped some 60 m at the end of the Oligocene. The combined effect of these three factors resulted in a depositional hiatus at the Oligocene–Miocene transition.
2.3.3 Neogene and Quaternary deposits
Subsidence in the North Sea Basin prevailed during the Neogene and Quaternary periods, enabling huge amounts of sediment supplied by the major river systems to be accommodated. The basin became filled along its margins, and a gradual but continuing shift of the coastline westwards and northwestwards resulted from the prograding fluvial systems.
2.3.3.1 Marine deposits
In the present onshore part of the Dutch territory, five marine formations are discerned within the Neogene–Quaternary sequence. They are briefly described here; for more details, see the nomenclature (https://www.dinoloket.nl/nomenclator). The Breda Formation consists of marine glauconitic fine sand and silty clay, and was deposited during the Miocene. The formation occurs everywhere in the subsurface, except in a zone parallel to the eastern border. Extremely quartz-rich sand (silica sand) occurs in South Limburg and in parts of the Roer Valley Graben: it is situated in between two layers of lignite. In the west of North Brabant, deposits very rich in glauconite (up to 70%) have been assigned to the Rucphen Member. Going south to north in the basin, the deposits of the Breda Formation tend to become finer-grained and their clay content increases.
Deposits of the Oosterhout Formation overlie the Breda Formation, and they consist mainly of fine- to medium-grained sand, clay and sand with abundant shell remains. The glauconite content is considerably lower than in the Breda Formation. The lower boundary is sharp in the southwestern part of the country but gradual elsewhere. Extremely shell-rich deposits (crags) occur in the west of North Brabant and have been assigned to the Sprundel Member. In the same region, a brown clay deposit marks the upper part of the formation and has been assigned to the Wouw Member. The formation is Pliocene in age and its offshore equivalent is called the Brielle Ground Formation.
The Maassluis Formation consists of fine- to coarse-grained shell-bearing sand sometimes alternating with layers of clay. The transition to the underlying Oosterhout Formation is gradual and difficult to define in the western and northern parts of the country. At the eastern and southeastern limits of its distribution, the deposits interdigitate with fluvial sediments of the Eridanos (i.e. the major rivier system that originated in the Baltic region and existed from Eocene to Middle Pleistocene; Overeem et al., Reference Overeem, Weltje, Bishop-Kay and Kroonenberg2001) or Rhine–Meuse river systems. The Maassluis Formation was formed during the first part of the Pleistocene.
At the end of the Early Pleistocene the marine realm had migrated to positions west of the present coastline of the Netherlands. This situation persisted for a long time, and not until the Saalian land ice cover had melted could the sea again transgress over the western part of the country. Fine- to coarse-grained shell-bearing sand and clay deposits formed during that marine phase have been assigned to the Eem Formation. Thick marine clay deposits may occur in the glacial basins formed during the Saalian advance of major ice sheets.
The uppermost marine deposits were formed during the Holocene transgression when the sea invaded the western and northern parts of the Netherlands and they have been assigned to the Naaldwijk Formation. This formation consists of interrelated estuarine, tidal and coastal deposits forming a seaward thickening wedge of sediment in the coastal plain. The lithological composition varies from fine- to medium-grained sand and clay deposited in mudflats, salt marshes and tidal lagoons. Along the present coastline the wedge of Holocene coastal deposits is bordered by thick accumulations of sand stored in natural barriers and coastal dunes.
2.3.3.2 Fluvial deposits
The geological evolution of the Neogene and Quaternary is characterised by the formation of huge deltaic systems, the uppermost sets of which consist of coarse-grained fluvial deposits. The latter may be up to 200 m thick in the onshore part of the Netherlands. The fluvial deposits are categorised into three major groups reflecting their source areas: the Rhine, Meuse and Eastern river systems (Baltic area). Smaller systems are recognised related to rivers draining central and north Belgium, including the Scheldt. There are 13 fluvial formations in total. During most of the Pliocene and Pleistocene the Netherlands was situated downstream of the confluence of Rhine and Meuse (Westerhoff et al., Reference Westerhoff, Kemna and Boenigk2008). Consequently the sediment has a mixed Rhine–Meuse composition. However, being the larger river the Rhine overprints nearly all sedimentary and mineralogical properties of these mixed deposits. The provenance of Meuse can only be identified in very coarse-grained components, such as gravel, stones and boulders derived from rocks in the Ardennes and northern France.
Rhine–Meuse
The first fluvial sediments formed in the Netherlands were deposited by a predecessor of the river Rhine system. This pre-Rhine had no connection with the Alpine area and its source material consisted of the strongly weathered overburden of the Rhenish Massif (Boenigk, Reference Boenigk2002; Schäfer et al., Reference Schäfer, Utescher, Klett and Valdivia-Manchego2005; Westerhoff et al., Reference Westerhoff, Kemna and Boenigk2008). As a result, the deposits of the pre-Rhine system are typified by a high percentage of quartz and the mineralogical composition is dominated by so-called stable heavy minerals such as tourmaline, staurolite and some metamorphic minerals. The deposits of the pre-Rhine system have been assigned to the lower situated Inden Formation and the overlying Kieseloolite Formation. The Inden Formation consists of medium- to coarse-grained grey sand with multiple remains of wood and lignite, and was formed during the Late Miocene. It occurs only in the southeastern part of the Roer Valley Graben. The Kieseloolite Formation consists of pale sand and gravel alternating with some local to regional clay layers. The latter often contain layers of brown coal or lignite. The unit is Late Miocene to Pliocene in age and is found on the Peel Block, Roer Valley Graben and parts of South Limburg.
As a result of the progressive enlargement of the pre-Rhine drainage basin towards southern Germany and the Alpine region, new source areas of metamorphic and crystalline rocks became available to the Rhine system during the second half of the Pliocene. Evidence for this in the sediments is the presence of mica flakes, an increase in red-brown particles (i.e. feldspar and sandstone) and a decreasing quartz content. However, most striking is the major shift in the heavy mineral composition: from nearly 90% predominance of stable heavy minerals (known from the Inden and Kieseloolite Formations) to a nearly 90% predominance of so-called unstable heavy mineral assemblages. The latter consist of minerals such as garnet, epidote, alterite/saussurite and hornblende (Boenigk, Reference Boenigk2002; Westerhoff, 2011).
The earliest deposits of the enlarged Rhine system have been assigned to the Waalre Formation and consist of fine- to coarse-grained grey sand alternating with clay layers up to several metres thick. The Waalre Formation was deposited during the Late Pliocene and Early Pleistocene. Due to the tectonic setting the upper part of the formation inclines towards the northwest. The Sterksel Formation is easily recognisable from its coarse-grained gravel-bearing basal part and stratigraphical situation above the Waalre Formation. Clay layers in the Sterksel Formation occur only locally. The relatively large amount of coarse material of the Sterksel Formation may result from accelerated uplift of the Rhenish Massif, which occurred at the onset of the Mid-Pleistocene (Garcia-Castellanos et al., Reference Garcia-Castallanos, Cloetingh and Van Balen2000). The formation was deposited during the latest part of the Early Pleistocene and the earliest part of the Mid-Pleistocene.
The uppermost three formations of the Rhine system are mineralogically characterised by a relatively high content of volcanic minerals, mainly augite, resulting from renewed volcanism in the Eifel region. The Urk Formation is a typical fine- to coarse-grained Rhine sediment, with a low gravel content and near absence of clay layers. It occurs in the central and northern parts of the country and was formed during the second half of the Mid-Pleistocene. It is overlain by the Kreftenheye Formation, a mixed Rhine–Meuse deposit covering the centre of the Netherlands. The lithology ranges from fine- to coarse-grained sand and some gravel. Locally, clay layers may be intercalated. In the central river district of the country, a typical silt-containing and compact grey clay layer occurs at the top of the formation. The layer has been lithostratigraphically assigned to the Wijchen Bed. The stratigraphically uppermost and youngest fluvial deposits in the Netherlands are known as the Echteld Formation. This is a mixed Rhine–Meuse deposit whose formation was strongly influenced by the Holocene sea-level rise. The meandering to anastomosing system supplied sediment consisting of fine- to medium-grained sand that was mainly deposited in channels and on levees. Grey to brown clay with plant and wood remains was deposited in extensive flood basins.
Meuse
All the pure Meuse deposits in the Netherlands have been assigned to the Beegden Formation. Generally, the deposits consist of medium- to coarse-grained sand and coarse gravel, stones, cobbles and boulders. Locally, clay layers with a limited lateral distribution may occur. More widespread is the silt-containing compact clay layer present at the top of the Late Weichselian fluvial deposits in north and central Limburg. This is the so-called Wijchen Bed that continues into the Kreftenheye Formation. Deposited by the Meuse, all fluvial terrace deposits in South Limburg form part of the Beegden Formation. The oldest terraces date from as far back as the Pliocene.
Eastern river systems
Sediment of eastern provenance was supplied by the so-called Eridanos river system that originated in the Baltic area (Overeem & Kroonenberg, Reference Overeem and Kroonenberg2002). The deposits have been assigned to the Peize Formation and consist of pale medium- to coarse-grained sand, rich in quartz, and some gravel. Some clay layers may occur, especially in the lower part of the unit. In the uppermost part of the formation, coarse gravel of Baltic provenance may occur locally: the so-called Hattem beds (Zandstra, Reference Zandstra1971). The formation covers the northern half of the Netherlands, is up to 150 m thick and interdigitates with the Waalre Formation in the central part of the country. The age ranges from Late Pliocene to Early Pleistocene.
The Appelscha Formation is the second unit of fluvial deposits of eastern provenance. However, most of the sediment originates from rivers draining North Germany and the Mittelgebirge. The sediment consists of medium- to very coarse-grained grey–white sand and gravel. The formation has a continuous distribution in the northern half of the country and some patchy occurrences in the eastern part of Overijssel. The deposits date from the latest part of the Early Pleistocene to the earliest part of the Mid-Pleistocene.
The Quaternary glaciations finally destroyed the hydrogeographical patterns in northern Europe and probably also destroyed the upper reaches of the Baltic river system. As a result, the supply of sediment from the Baltic region and the Mittelgebirge to the Netherlands ceased.
Belgian rivers
During the Late Pliocene and entire Early Pleistocene, the central and northern part of Belgium was drained by a series of rivers flowing northeast, which supplied fine- to medium-grained pale sand to the southern part of the Netherlands. Their deposits have been assigned to the Stramproy Formation. The sediment is rich in quartz and the heavy minerals are predominately stable assemblages. Clay layers are only 1–2 m thick in the southern part of the distribution area but may be several metres thick in the more westerly areas. Deposits of the Stramproy Formation interdigitate with the Waalre Formation in the Roer Valley Graben and the area west of it. Furthermore, deposits of the Stramproy Formation overlie the Waalre Formation in large parts of the southern and western Netherlands.
Regional tectonics and the development of west to east running cuestas during the Middle Pleistocene caused a restructure of the drainage pattern of the rivers in northern Belgium. The rivers changed their northeastern running courses to western ones and became confluent with the Scheldt River. Fluvial deposits of this Scheldt system consist of fine- to moderate-grained sand and are found in the southwestern part of the Netherlands and assigned to the Koewacht Formation. The Koewacht Formation was formed during the Middle Pleistocene. Holocene deposits of the Scheldt consist of fine-grained sediment very rich in organic matter (peat and gyttja) that is found in the paleovalley of the Scheldt in the southwestern part of the coastal plain. These specific deposits are assigned to the Kreekrak Formation.
2.3.3.3 Glacial deposits
The Quaternary sequence of marine and fluvial deposits in the Netherlands was interrupted at least twice by a major southward advance of the Scandinavian ice sheets. The first time was during the Elsterian glaciation some 400,000 years ago. The glacial deposits formed during this period have been assigned to the Peelo Formation, which occurs only in the northern part of the country and consists of fine- to coarse-grained sand and stiff dark brown clay. The sand is a meltwater deposit partly formed in deeply incised (more than 500 m!) tunnel valleys in which clay is often the uppermost part of the sedimentary infill. The clay is often described as ‘Potklei’ and nowadays lithostratigraphically assigned as the Nieuwolda Member of the Peelo Formation.
Glacial deposits formed during the Saalian ice coverage of the Netherlands all form part of the Drente Formation. The ice sheet extended to approximately the imaginary Amsterdam–Nijmegen line, and at its margins ice-pushed ridges and glacial basin were formed. Three main lithostratigraphical units subdivide the Drente Formation, two of which will be considered in this manuscript (Gieten and Uitdam Members; Table 1). The third unit is the Schaarsbergen Member, which is composed of fine- to coarse-grained sand and gravel formed as glacio-fluvial meltwater deposits and sandr-type deposits.
2.3.3.4 Other deposits
A separate category of lithostratigraphical units consists of material resulting from intense weathering, aeolian deposits, sediment formed by small-scale fluvial systems, and large-scale peat formation in extensive swamps, coastal plains and alluvial floodplains. Three units consisting entirely of peat are discerned in the Neogene–Quaternary sequence. The lowermost is called the Ville Formation (Schäfer et al., Reference Schäfer, Utescher, Klett and Valdivia-Manchego2005): it is the main brown coal formation in the lower Rhine Embayment. Two wedges of the Ville Formation extending westwards reach the Netherlands and are situated below and above the well-known silica sand of the Heksenberg Member in South Limburg.
Peat formed in the Eemian coastal plain has been assigned to the Woudenberg Formation and occurs mainly in a zone bordering the marine Eem Formation in the Gelderse Vallei, a former glacial basin. Extensive Holocene peat formation occurred in the coastal plain, the fluvial district and in regionally poorly drained areas such as the till-dominated region in the northern Netherlands. All these peat accumulations have been assigned to the Nieuwkoop Formation. It is worth noting that very local and non-mapable peat accumulations that may have formed in a flood basin or a local dune valley are not classified as part of one of these main peat formations. Instead, the peat layers are interpreted as local phenomena and genetically related to the general lithological composition of a formation.
The Holset Formation consists of fine-grained sand and blocks of sandstone resulting from early diagenesis. The formation occurs only in South Limburg and exclusively in areas not covered by Pliocene and Pleistocene fluvial deposits. The distribution of the Heyenrath Formation is also restricted to South Limburg. The deposits consist of weathered flint in a matrix of clay and result from weathering of the underlying chalk. They have an irregular patchy distribution and the thickest accumulations occur in karst cavities like ‘organ pipes’.
The most important group of deposits in this category is formed by the Boxtel Formation (Schokker et al., Reference Schokker, Weerts, Westerhoff, Berendsen and Den Otter2007). It consists of fine- to medium-grained sand, clayey loam and locally formed gyttja. The deposits result mainly from aeolian processes or small-scale fluvial activity under changing or cool climatic conditions. The age ranges from Mid-Pleistocene to Holocene. The deposits of the Boxtel Formation cover almost the entire country except for the fluvial district and the coastal plain, where they have been removed by tidal erosion during the Holocene. Nine members are discerned within the Boxtel Formation, four of which (Liempde, Schimmert, Tilligte and Wierden) will be considered in this manuscript because there is specific data available for these members (Table 1). It is worth noting that a large part of the Boxtel Formation has not been subdivided into separate members. This mainly concerns sediment formed by small-scale fluvial systems under periglacial conditions.
3 Review procedure and methodological approaches
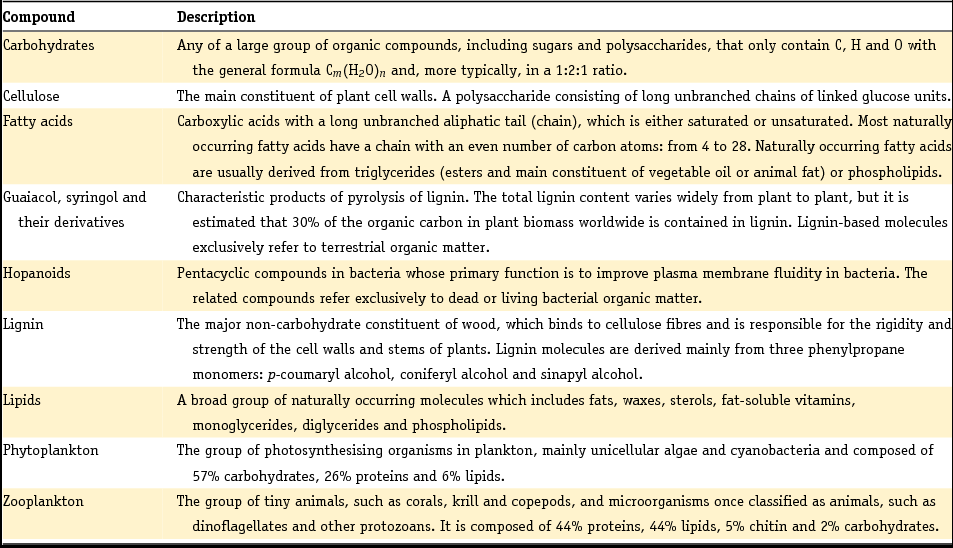
This section covers a few general topics. First, we present the general procedures we followed when carrying out this review and describe how we delimited the scope of the review. Second, we discuss the methodological approaches used to characterise Dutch sediments by their mineralogy and grain size distribution. It is important to summarise these approaches because this makes it easier to understand the insights presented in subsequent sections and in the original literature. Third and last, we present Table 2 in which we briefly describe the types of organic compounds and plankton groups as discussed in the organic geochemistry sections of the current review. This should serve the reader who is not very familiar with organic geochemistry. Further, we will refer to OM when discussing bulk organic matter. We use POC exclusively to refer to particulate organic carbon associated with suspended matter.
Table 2. Description of organic geochemical compounds and types of plankton as mentioned in this manuscript (basically derived from http://www.thefreedictionary.com/ and checked against Engel & Macko (Reference Engel and Macko1993)).

3.1 Scope of the review
Since we intended to summarise direct evidence on the mineralogy of sediments, we focused our review on research that directly studied samples of sediment or suspended matter. We considered peer-reviewed literature (including PhD theses) as the prime source for individual topics. If we were unable to find any peer-reviewed literature on a topic, we turned to reports by Dutch research institutes, MSc theses or publications in professional Dutch journals.
Over the years, several geological lithostratigraphical units have been defined and used in the Netherlands and therefore older articles sometimes refer to geological formations that are not used in the current lithostratigraphy. When this occurred, we adapted the classification to the present-day one, always striving to avoid ambiguity. This was particularly the case for the Tegelen Formation, which was intensively studied by Huisman and co-workers and is nowadays classified as the Waalre Formation (Huisman et al., Reference Huisman, Klaver, Veldkamp and Van Os2000; Westerhoff, Reference Westerhoff2009). In other cases, reference is made to both the former and the present lithostratigraphical unit. An example is the Twente Formation, which became part of the much broader and sedimentologically heterogeneous Boxtel Formation (Schokker, Reference Schokker2003).
We have focused on the natural background mineralogy of Dutch sediments. However, this is almost impossible with respect to recent sediments, largely because the present-day massive hydraulic infrastructure resulting from Dutch water management has probably also affected the mineralogy and geochemistry of sediments deposited in recent centuries. The clearest examples of impact on sediment geochemistry are the closure of the saline Zuiderzee and several estuaries in the southwestern Netherlands, and the subsequent creation of freshwater lakes such as Lake IJsselmeer and Haringvliet. Also relevant are changes in the sediment load of the Rhine and Meuse water systems resulting from canalisation and land use changes (Middelkoop et al., Reference Middelkoop, Erkens and Van der Perk2010), as well as anthropogenic increases in dissolved major ions such as sulphate (Griffioen et al., Reference Griffioen, Passier and Klein2008). The intensity of sulphide production in association with mineralisation of organic matter is to some extent related to the anthropogenic contamination of surface and groundwater with sulphate. We will characterise the early diagenetic reactions that are associated with the major elements (including PO4) and also the impact of regional, palaeohydrological processes on the geochemistry of buried sediments as far as possible. We will not consider the anthropogenic contamination of soil, aquatic sediment and suspended matter with trace metals or organic microcontaminants. Neither do we review the impact of soil chemistry processes related to pedogenesis, except for decalcification, pyrite oxidation and impact of weathering on heavy mineral assemblage. We pay attention to the major organic geochemistry of bulk organic matter (OM) in the riverine and estuarine systems, where – especially in the Scheldt system – there has been severe anthropogenic pollution with organic waste. Our justification is that OM can be a major reactant in these surface water systems, where it must be remembered that due to pollution the concentration of organic matter may be higher under present-day conditions than was the case in the past under natural conditions.
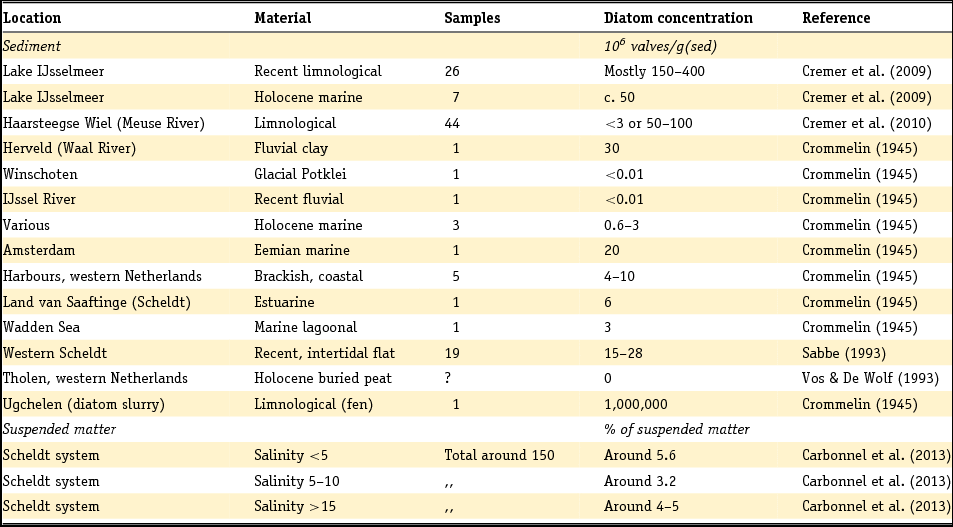
Recent sediments in large lakes that form an integral part of the Rhine and Meuse water system (in particular Haringvliet, Hollands Diep and Lake IJsselmeer) are discussed in the section on fluvial deposits because their composition is strongly controlled by the sediment load of these major rivers. Lacustrine sediments deposited in other, more isolated lakes will be discussed separately: we will deal with fens in the Pleistocene part of the Netherlands or palaeolakes as mineralogical information is present for these.
3.2 Grain size distribution
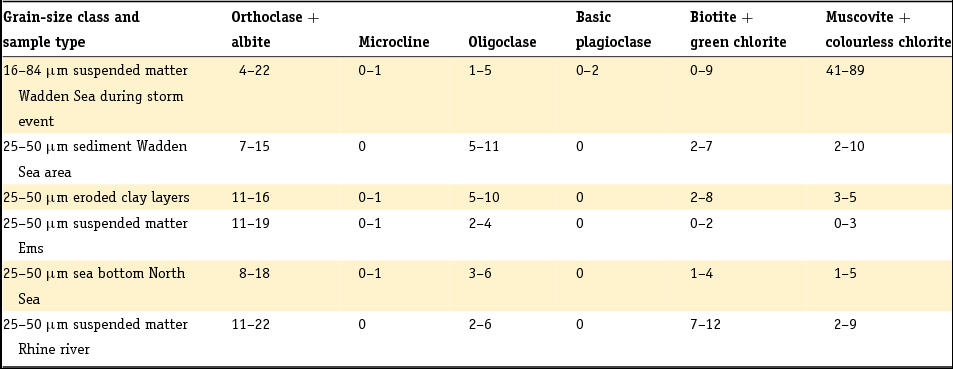
There is a general relationship between grain size distribution and mineralogy because minerals in clastic sediments have a typical range in grain size related to their hardness (e.g. Boggs, Reference Boggs2009). We therefore pay attention to this relationship as it is observed for Dutch sediments. Grain size analyses before about 1990 are based on sieve-and-pipette analysis whereas nowadays laser diffraction analysis is performed routinely. The two fundamentally different techniques yield different results, with different discrepancies systematically found for different sediment types (Konert & Vandenberghe, Reference Konert and Vandenberghe1997; Buurman et al., Reference Buurman, Pape, Reijneveld, De Jong and Van Gelder2001). However, correlations for summed fractions are generally better than those for individual size fractions because of the integration among classes and limited shifts between classes. As a rule of thumb, the laser diffraction fraction of <8 μm is equivalent to the classic clay fraction <2 μm found by the pipette method (Konert & Vandenberghe, Reference Konert and Vandenberghe1997).
In the past, the 10 or 16 µm grain size was frequently taken as the boundary between clay and silt or the < 16 µm fraction was denoted with the Dutch word ‘slib’, which translates into English as silt or mud (Crommelin, Reference Crommelin1943; Egberts, Reference Egberts1950; Favejee, Reference Favejee1951; Van Straaten, Reference Van Straaten1954; Pons, Reference Pons1957; Verhoeven, Reference Verhoeven1962; De Groot, Reference De Groot1963). More recently, but still following the convention based on sieve-and-pipette analysis, the silt fraction has been defined as the 2–50 (Dutch soil classification; e.g. Van der Sluijs & Locher, Reference Van der Sluijs, Locher, Locher and De Bakker1990) or 2–63 μm fraction (NEN 5104, 1989). Note that the latter fraction is most commonly used in geology. We will follow this definition and refer to the <16 μm fraction as the ‘fine fraction’ in addition to the clay fraction and the silt fraction. The term ‘mud’ has frequently been used within the context of marine or estuarine sediments. It denotes soft, clay-rich deposits with high contents of water. We will use this term in this context.
A general remark must be made when combining grain size analyses and chemical analyses. Carbonates and natural organic matter are mostly removed prior to grain size analysis. Thus grain size analyses refer to the silicate components of the sediment and some other minerals, and are not representative for the solid matrix as a whole. Moreover, different size classes are systematically underestimated because particulates that are associated with these size classes have been removed. This holds, for example, for the silt class in which Ca carbonates are commonly found (see later). A major proportion of the particulates may be removed, particularly in the case of suspended matter. Several studies point out that organic matter, carbonates and Fe colloids may account for up to 60% of the suspended matter (De Groot, Reference De Groot1963; Wartel, Reference Wartel1977; Van Eck, Reference Van Eck1982a; Van de Meent et al., Reference Van de Meent, De Leeuw, Schenck and Salomons1985): the highest fraction of 60% was found for autochthonously produced biomass in the northern part of Lake IJsselmeer at the end of 1970s when the lake was highly eutrophic. This was probably an extreme, but 10–30% will not be uncommon.
3.3 Clay minerals analysis
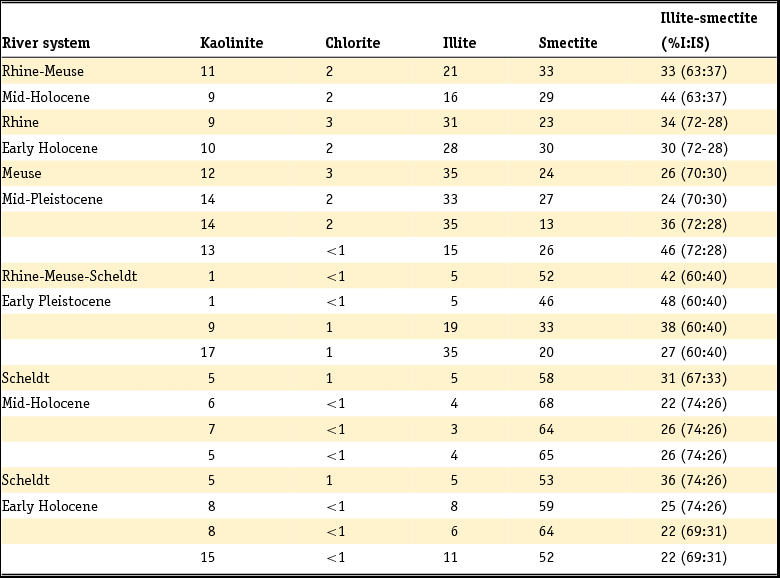
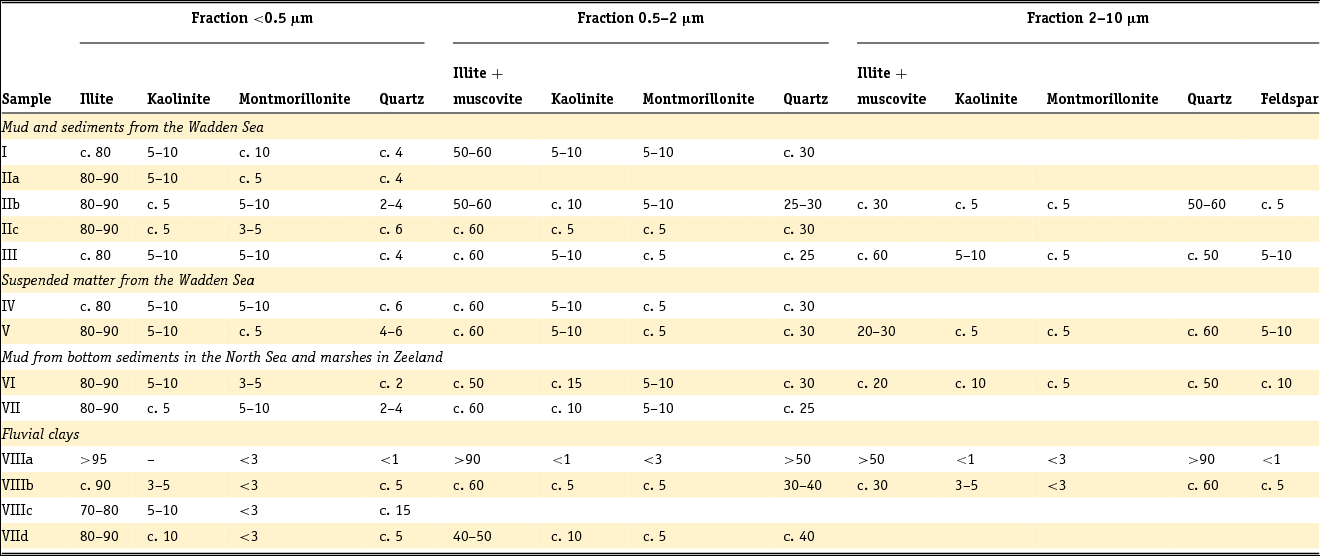
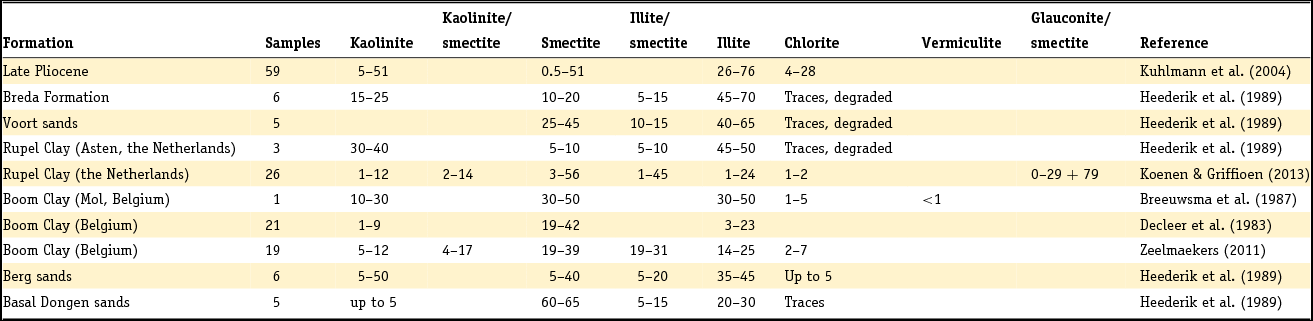
Clay minerals are often said to be the garbage bags of sediments and do not have fixed compositions in nature. As early as 1939, Favejee (Reference Favejee1939) quantified the amount of the clay minerals illite + muscovite, kaolinite, montmorillonite and quartz as abundant, less abundant, few and not present using X-ray diffraction (XRD) analysis. This technique has evolved during recent decades into more quantitative approaches, and it is worth noting that we are not aware of any published clay mineralogy study in which the accuracy of analysis is quantitatively indicated. The clay mineralogy of Dutch sediments as reported by Favejee (Reference Favejee1939), Breeuwsma (Reference Breeuwsma1985), Huisman (Reference Huisman1998) and Tebbens et al. (Reference Tebbens, Veldkamp and Kroonenberg1998) was determined at Wageningen University. The methods and equipment used evolved with time and here we first describe the method reported in the PhD theses of Huisman (Reference Huisman1998) and Tebbens (Reference Tebbens1999), and give the abbreviations for the other Wageningen studies as far as they are described.
Standard powder XRD analyses were performed on freeze-dried or air-dried samples. Clay mineralogy was determined by first treating the samples with Na acetate, H2O2 and Na dithonite to remove carbonates, organic matter and Fe and Mn oxyhydroxides. Subsequently, the clay fraction was separated by pipetting after settling, and X-ray diffractograms were made of oriented samples. Tebbens saturated his samples not only with 1 M KCl but also with 1 M MgCl2 and glycerol (these latter saturations are not mentioned in Huisman). The KCl-saturated samples were additionally heated at 150°C. The swelling characteristics were determined via XRD measurements of the 001 reflections. The diffractograms were digitised by hand. Semi-quantitative peak surfaces were obtained by multiplying the 0.7 (kaolinite), 1.0 (illite), 1.4 (chlorite and vermiculite) and 1.8 nm (smectite with glycol in its interlayer) peak heights above the background with the peak widths at half-peak heights. According to Tebbens (Reference Tebbens1999), reliable relative clay percentages can then be calculated within one dataset by dividing the peak surface for an individual clay mineral by the sum of all peak surfaces. Both Huisman and Tebbens also used the major and trace element content of the clays in the interpretation of the clay minerals. The method of Breeuwsma is similar to the method of Tebbens but he did not saturate his samples with KCl and measured his air-dried samples also after heating them to 300 and 500°C. The 300°C diffractograms were used to distinguish vermiculite and chlorite. Breeuwsma used not only the XRD results but also the K2O content (illite), K-fixation (vermiculite), cation exchange capacity (CEC; smectite) and loss on ignition (chlorite) of the clays for the presence of a mineral. Irion & Zöllmer (Reference Irion and Zöllmer1999) made their XRD analyses of the North Sea sediments without the removal of carbonate, organic matter and iron. In order to obtain a standard condition for cation fixation and for a better recognition of the minerals, the <2 µm fraction on the smear slides was treated with Mg acetate, K acetate and the vapour of ethyleneglycol at 80°C. Only the four common main groups of clay minerals, smectite, illite, kaolinite and chlorite, could be distinguished. Semi-quantitative contents for each group were obtained on the basis of weighted peak areas.
The quantitative method used by Zeelmaekers (Reference Zeelmaekers2011) and Adriaens (Reference Adriaens2014) is extensively described by the first and is at present the state-of-the-art methodology for how quantitative clay mineral analyses should be made. Preparation of the samples for the XRD, however, is similar, as is described above. The major difference is that the samples are saturated with Ca (1 M CaCl2) after the selective leaching of the samples to remove the carbonate, organic matter and the Fe oxyhydroxides. Oriented samples are made by sedimentation slides because they produce overall higher quality diffraction patterns than smear mounts. Routinely, the XRD recordings are recorded in three different states: air dry, ‘glycolated’ and heated at 550°C for 1 hour. These recordings are used to identify the main clay groups (smectite, illite, kaolinite and vermiculite). Ambiguities can be resolved by analysing oriented slides in additional cationic forms such as Mg and K (vermiculite) and Li (montmorrillonite as smectite species). The main difference with the Wageningen method is the characterisation of mixed-layered phases by modelling of the oriented slide diffraction patterns. For this modelling the Sybilla program (Chevron proprietary software) is used. It is designed to calculate theoretical XRD patterns of the basal reflections of discrete and mixed-layered clay minerals, and to combine them to model the compositions of experimental diffraction patterns. The use of independent control parameters such as CEC and chemistry serves as an additional support for the accuracy of the method.
3.4 Reactive Fe minerals
Reactive Fe minerals are important constituents of sediments and they have been studied in Dutch sediments in six different ways. First, concretions or small associations of pyrite, siderite (which after exposure to air changes colour from white to yellow), vivianite (changes from white to blue after air exposure) and Fe oxides together with coatings (particularly of Fe oxyhydroxides) have been observed with the naked eye in sediment samples. Second, a few optical and electron microscopy studies have been performed and it is worth noting that these have identified several Fe oxides appearing as opaque minerals in reflecting light under the microscope (goethite, magnetite, hematite, ilmenite and chromite). Third, selective extractions have been performed, in which extracted amounts have been interpreted as solid compounds such as acid volatile sulphides or amorphous Fe hydroxides. It must be remembered that other minerals also dissolve when natural soil or sediment samples are subjected to extraction techniques (e.g. Fe-bearing Al silicates; Griffioen & Broers, Reference Griffioen and Broers1993), therefore assuming that the operationally extracted amounts equal the true mineralogical contents can be a slight to gross simplification, depending on the mineralogical composition of the sediment analysed. Fourth, total elemental analysis is used to calculate solid compounds, making basic assumptions about the presence of individual minerals and neglecting other minerals. This is used in particular to determine pyrite content in aquifer sediments, where pyrite is calculated from total sulphur and the associated Fe is a fraction of total Fe. Fifth, XRD is used to identify Fe minerals if these are present in substantial amounts. This technique is especially powerful for siderite, which is difficult to identify by means of other routine techniques. Finally, Hartog et al. (Reference Hartog, Griffioen and Van der Weijden2002), Hyacinthe & Van Cappellen (Reference Hyacinthe and Van Cappellen2004) and Zhang et al. (Reference Zhang, Slomp, Broers, Bostick, Passier, Bottcher, Omoregie, Lloyd, Polya and Van Cappellen2012) used unique, specialised techniques, which will be mentioned in the following sections.
Geochemical analyses have increasingly been used to deduce which reactive Fe minerals are present and in which amounts. Reactive Fe is defined as the fraction of Fe that is not present in Al silicates. This means that the reactive Fe minerals are considered to be Fe sulphides, Fe oxyhydroxides, siderite, Fe phosphates and also the clay mineral or mica glauconite. Glauconite is an Fe-rich mica of marine diagenetic origin, with the general formula (K,Na,Ca)1.2–2(Fe,Al,Mg)4(Si7–7.6Al1–0.4O20). Jarosite in acid sulphate soils might also be a reactive Fe mineral, since it is formed by pyrite oxidation. Huisman & Kiden (Reference Huisman and Kiden1998) and Dellwig et al. (Reference Dellwig, Watermann, Brumsack, Gerdes and Krumbein2001, Reference Dellwig, Bottcher, Lipinski and Brumsack2002) proposed calculating reactive Fe as the difference between total Fe and Fe bound to unreactive minerals such as Al silicates, ilmenite or possibly chromite. Unreactive Fe is empirically estimated as one quarter of total Al. Subsequently, total reactive Fe is calculated as the difference between total Fe and unreactive Fe:
FeTR = 2 × MFe/MFe2O3 × [Fe2O3 – 0.25Al2O3]
where FeTR is total reactive Fe, Mi refers to molar mass of compound i, and Fe2O3 and Al2O3 are total Fe and Al contents, respectively, expressed on an oxide basis.
It is worth pointing out that the empirical approach is in conflict with the findings of Canfield et al. (Reference Canfield, Raiswell and Bottrell1992), who deduced half-lives of sedimentary iron minerals with respect to sulphidation. They concluded that the half-life for sheet silicates is 84,000 years, which is within the time frame of the Pleistocene period. Weathering of Fe-Al silicates may lead to reactive Fe minerals, dissolved ferrous Fe and secondary kaolinite, or a secondary clay mineral that contains Fe mobilised by this weathering. This means that the empirical relationship may be useful for Holocene sediments, but might be more questionable for sediments of Middle Pleistocene age and older. Here, the conclusion of Canfield et al. (Reference Canfield, Raiswell and Bottrell1992) is probably a simplification too. It is also worth mentioning that in the 19th century Van Bemmelen (Reference Van Bemmelen1863) proposed the concept of a fixed Al2O3 to Fe2O3 ratio in silicates of clay: the Fe2O3 content is about 7% when the Al2O3 content is 15–20% and it is 2.5% at the transition between clay and sand.
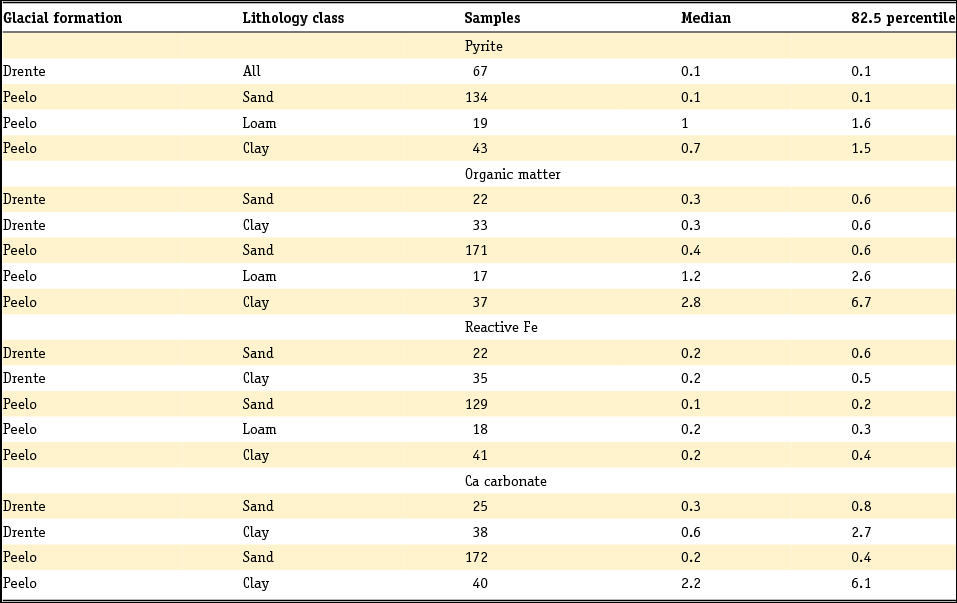
In a subsequent step, pyrite-bound Fe can be isolated from the total amount of reactive Fe. Pyrite content and pyrite-bound Fe are often calculated from the total S content for clastic sediments, where total S content is assumed to represent pyrite (Van Gaans et al., Reference Van Gaans, Griffioen, Mol and Klaver2011; Griffioen et al., Reference Griffioen, Klein and Van Gaans2012):
and
where FeS2 is pyrite content, Fepy is pyrite-bound Fe and S is total sulphur. By subtracting pyrite-bound Fe, the non-pyrite bound reactive Fe (Fereac) can be calculated:
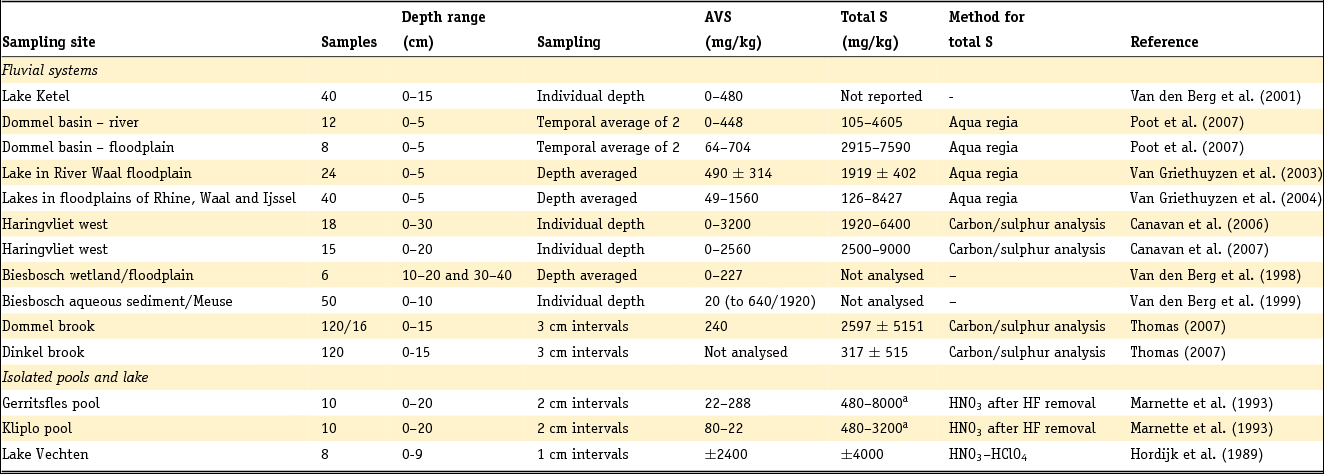
These formulae work well as long as total reactive Fe is higher than total S, taking into account stoichiometry. The presence of organic S, gypsum or other Fe sulphides is neglected. For Dutch conditions, this holds for sediment below a few metres depth and other than peat, but not for samples from sediment in which early diagenesis or pedogenesis is ongoing, i.e. samples from recent sediments or soils. Note that international field studies on S speciation have revealed that iron sulphides other than pyrite are usually negligible in different kinds of sedimentary groundwater settings (e.g. Bates et al., Reference Bates, Spiker and Holmes1998; Massmann et al., Reference Massmann, Pekdeger and Merz2004; Chambers & Pederson, Reference Chambers and Pederson2006; Jakobsen & Cold, Reference Jakobsen and Cold2007; Schwientek et al., Reference Schwientek, Einsiedl, Stichler, Stoegbauer, Strauss and Maloszewski2008). Interpretation of incubation experiments based on reaction stoichiometry also indicates that pyrite is present as a reductant in various Dutch aquifer sediments (Hartog et al., Reference Hartog, Griffioen and Van der Weijden2002, Reference Hartog, Griffioen and Van Bergen2005; Van Helvoort et al., Reference Van Helvoort, Griffioen and Hartog2007). Further, the presence of S as barite is negligible, where Ba is dominantly present in K-feldspar (Klein & Griffioen, Reference Klein and Griffioen2010). Oenema et al. (Reference Oenema1990a,b) and Hartog et al. (Reference Hartog, Griffioen and Van der Weijden2002) used HNO3 extraction combined with total S analysis as a more direct method to determine the pyrite content, where the molar Fe to S ratio was close to 0.5. Regrettably, this method has not been applied more often. For recent, aquatic fresh sediments, the pyrite content has been calculated as the difference between total S and acid volatile sulphide (AVS) content, where total S has been analysed by either CS elemental analyser or aqua regia destruction and ICP analysis. AVS has often been assumed to equal mackinawite (FeS), greigite (Fe3S4) and dissolved sulphides (when pore water was included in the analysis), which are also precursors for pyrite. These assumptions have been heavily criticised by Rickard and Morse (Reference Rickard and Morse2005). The Dutch data for the individual aquatic sediment samples in fresh environment show that the AVS content is often considerably smaller than the total S content, as summarised in the next section. This means that most of the total S will indeed be pyrite, also because the contribution of organic S is probably small for samples that typically contain several to 10% of organic carbon. For estuarine environments, Oenema (Reference Oenema1990a,b) found that AVS can approximately equal HNO3 extractable pyrite-S. This means that AVS cannot be neglected relative to total S for these sediments but leaves open the question of what AVS actually represents in this case.
3.5 Carbonate analysis
The carbonate content of sediments is an important characteristic. Different analytical techniques have been used to characterise the carbonate content of Dutch sediments. The most common ones nowadays are CS elemental analysis and thermogravimetric analysis (TGA). In CS elemental analysis, carbonate is measured after removal of organic matter or calculated as the difference between total C and organic C. The latter may have a substantial relative error when two large numbers are subtracted from each other. In both cases, it is mostly assumed that all carbonate is calcite or aragonite and not dolomite or siderite, which is not self-evident. The same implicit assumption is made in TGA analysis, where carbonate is commonly calculated from the weight loss between 550°C and 800°C. Other processes may interfere in this trajectory, such as loss of S from pyrite, or loss of crystal water from the clay minerals kaolinite and montmorillonite (Guggenheim & Koster van Groos, Reference Guggenheim and Koster van Gross2001; Roskam et al., Reference Roskam, Klaver and Griffioen2008). The representativeness of these analyses thus depends on the mineralogical composition of the sample. Marine sediments are generally most complicated because of the co-occurrence of organic matter, carbonates and Fe sulphides. Ca carbonate content has also been calculated from total Ca, based on X-ray fluorescence (XRF) and assuming a baseline between total Ca and total Al that represents Ca feldspar and Ca,Al silicates (Bakker et al., Reference Bakker, Kiden, Schokker and Griffioen2007):
The role of exchangeable Ca also deserves attention. Note that for a clayey sediment having a CEC of 50 meq/100 g and a Ca occupancy of 80%, the CaO content is 1.1%. If the relationship was established for a fresh groundwater environment in which Ca was the dominant cation, exchangeable Ca will also be included in the baseline. The relationship may then be less useful for groundwater environments dominated by Na and Mg. Otherwise, exchangeable Ca goes at the expense of Ca carbonate.
Verhoeven (Reference Verhoeven1962) presents three extraction methods for studying the co-occurrence of dolomite with calcite and aragonite, which gave some agreement among them: (1) difference in selective extraction between mild acetic acid and strong chloric acid, where the first extraction is assumed to represent calcite and aragonite and the latter also includes dolomite, (2) analysing Mg soluble in 8% HCl and correcting for exchangeable Mg and (3) kinetic analysis of the CO2 production during Scheibler analysis. The first method has been the most commonly used in the past.
4 Fluvial and alluvial sediments
Fluvial sediments have been deposited almost continuously since the Pliocene in the Netherlands. As shown in Fig. 4, a series of six formations is distinguished for the Rhine sediments whereas two formations (the Peize and Appelscha Formations) are distinguished for the northeastern Eridanos and North German systems. The Meuse system is associated with a single formation: the Beegden Formation. Two less extensive regional river systems are the Scheldt system in the southwest of the Netherlands and the Ems system in the northeastern corner of the Netherlands. In addition to these large river systems, there are alluvial sediments that form part of the heterogeneous geological formations of Boxtel (local drainage systems) and Stramproy (a river system draining northeast from Belgium).
4.1 Heavy minerals
4.1.1 Heavy mineral provinces
Heavy minerals have been intensively studied in the Netherlands in order to elucidate the stratigraphy, provenance and paleogeography of Dutch sediments. They are therefore dealt with first in this section. Edelman (Reference Edelman1933) was the first to study the mineralogical composition of the Dutch Quaternary deposits by examining sand-sized minerals both qualitatively and quantitatively. His classification of the heavy mineral associations resulted in so-called mineralogical provinces. Each province had its specific composition that resulted from the source(s) of supply. Care was therefore taken to establish the associations of major components and their specific appearance. Edelman also paid attention to numerical variations, distinguishing between ‘freak percentages’, changes in the geological hinterland and the interference of mineral provinces. He distinguished six sediment–petrological provinces (Table 3; Edelman, Reference Edelman1933), which were later expanded to eight provinces (Edelman, Reference Edelman1934; Van Baren, Reference Van Baren1934). Two groups were considered to be the on-shore continuation of the marine Young Tertiary/Quaternary A group (see section 5.1.1), both having a northern Scandinavian origin:
• A group with hornblende, epidote and garnet predominating, present in ‘marine and glacial’ deposits.
• X group with epidote and rutile predominating, present in ‘fluvio-glacial’ deposits.
Table 3. Standard heavy mineral composition (in counts of 100) determined in the original six heavy mineral assemblages distinguished by Edelman (Reference Edelman1933).

The other six groups belong to the so-called terrestrial B association, with a southerly to easterly provenance:
• B Scheemda group with the metamorphic heavy minerals kyanite, staurolite, andalusite and sillimanite predominating (from rivers from the Baltic area).
• B Saussurite group with saussurite and epidote, and in places augite predominating, from the older Pleistocene Rhine river system. In the literature, often referred to as the Rhine S association.
• B Lobith group (Edelman Reference Edelman1934) similar to the B Saussurite group but with abundant augite, from ‘recent’ Rhine material (see also Van Andel, Reference Van Andel1950), the augite originating from volcanic rocks in the Eifel area. In the literature, often referred to as the Rhine AS association.
• B Limburg group, in which the metamorphic minerals are characteristic and stable minerals (e.g. tourmaline, zircon) with a southerly provenance are abundant.
• B Elsloo group with brown-green amphibole predominating, with a southerly provenance.
• B Eijsden group (van Baren, Reference Van Baren1934) with abundant stable minerals and the Meuse chloritoid, representing the present-day Meuse sands.
4.1.2 Heavy mineral associations in fluvial formations
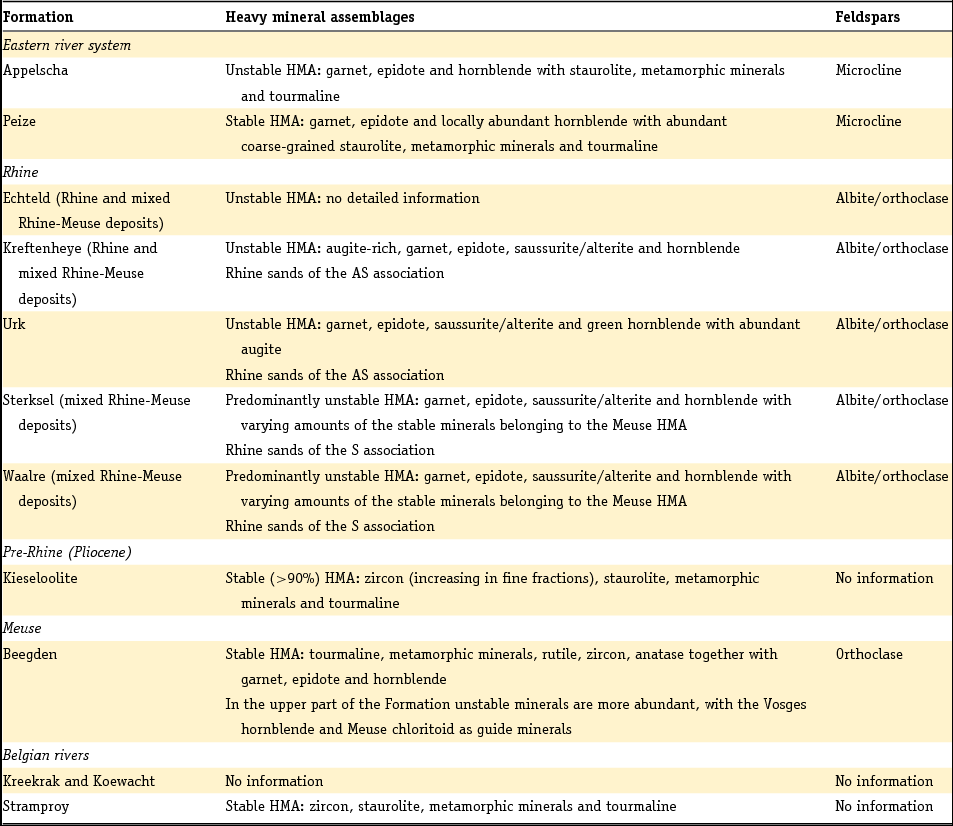
The method developed by Edelman was adopted by later researchers, but his sedimentary petrological provinces, defined as a group of sediments which constitute a natural unit in terms of age, origin and distribution, were later only used to derive paleogeographic conclusions or the origin of given sediments, as they do not enable sediments to be dated unequivocally unless the principle of superposition can be used (Zonneveld, Reference Zonneveld1958). A concept entirely different from that of the province is the mineral association: the particular combination of minerals that characterise a sediment. Essentially, the combination of minerals that characterises a sediment comprises two associations: a stable association (of minerals such as zircon, rutile, anatase and tourmaline) with/or without the metamorphic group index minerals (staurolite, kyanite, chloritoid, sillimanite and andulusite) and an unstable association (as well as epidote and garnet, also (and often dominantly) alterite and saussurite). In Table 4 the Dutch fluvial Pleistocene and Holocene formations (De Mulder et al., Reference De Mulder, Geluk, Ritsema, Westerhoff and Wong2003; Ebbing et al., Reference Ebbing, Weerts and Westerhoff2003) are classified in stable and unstable associations according to the above division. The three youngest formations of the Rhine system are in addition to the unstable heavy mineral association characterised by a relatively high content of volcanic minerals, mainly augite, resulting from renewed volcanism in the Eifel region. Guide minerals for the Meuse are the presence of Vosges hornblende and the specific Meuse chloritoid and garnet and for the Baltic sediments the presence of sillimanite.
Table 4. Heavy mineral and feldspar assemblages determined in the Dutch Quaternary fluvial deposits. Data for the heavy minerals are taken from Zonneveld (Reference Zonneveld1958), Doppert et al. (Reference Doppert, Ruegg, Van Staalduinen, Zagwijn, Zandstra, Zagwijn and Van Staalduinen1975) and Westerhoff (Reference Westerhoff2009). Feldspar data are from Van Baren (Reference Van Baren1934).

HMA, heavy mineral assemblage.
Table 3 illustrates that mineralogical analysis can reveal the provenance of a fluvial sediment, particularly when combined with superposition. However, when rivers coalesce or when a river has reworked the deposits of another river, the resulting heavy mineral composition depends on the volumes of sediment from each river and their respective heavy mineral fraction. From the point of confluence of the Rhine and Meuse onwards and also when the Meuse system reworked Rhine deposits during the Pleistocene or Holocene, the Meuse signature in heavy mineral composition fades rapidly, obscured by locally incorporated Rhine sediment. Some samples, for example, contain plenty of Eifel volcanic minerals that indicate Rhine provenance, but also show relatively high percentages of staurolite, metamorphic minerals and tourmaline, and have an overall low heavy mineral concentration that is atypical for Rhine sediments. When, in such cases, Rhine provenance is further contradicted by indications from other proxies such as detrital carbonate content (low: Meuse; high: Rhine) and flint within the gravel fraction (high: Meuse; low: Rhine-Meuse; absent: Rhine), the deposits studied are interpreted as having most recently been reworked by a Meuse system (Zonneveld, Reference Zonneveld1947, Reference Zonneveld1958; Busschers et al., Reference Busschers, Kasse, Van Balen, Vandenberghe, Cohen, Weerts, Wallinga, Johns, Cleveringa and Bunnik2007). For example, when the rivers Rhine and Meuse coalesced during the Pleistocene or Holocene, the influence of the Rhine sediments (the larger river) predominates over the influence of the Meuse. This effect is even more important in the resulting heavy mineral association (HMA) because Rhine sediments contain a much larger fraction of heavy minerals than the Meuse sediments (Zonneveld, Reference Zonneveld1958). In Rhine deposits reworked by the Meuse, it is important to detect the Meuse signature in order to be able to infer that such deposits were last reworked by the Meuse river system (Zonneveld, Reference Zonneveld1947; Busschers et al., Reference Busschers, Kasse, Van Balen, Vandenberghe, Cohen, Weerts, Wallinga, Johns, Cleveringa and Bunnik2007).
Another example of how HMAs can be used to unravel the complex Late Pliocene and Early Pleistocene fluvial history of the Netherlands and the southern North Sea Basin is illustrated by Westerhof (Reference Westerhoff2009). In the southern part of the Netherlands, fluvial deposition took place (the Rhine, Meuse and the northward-draining Belgian rivers), whereas in the central and northern part of the Netherlands, the Eridanos fluvial-deltaic system was important. During the Early Pleistocene, the Rhine-Meuse river system deposited a small lobate delta within the vast fluvio-deltaic Eridanos system. The deposits of both systems interdigitate in the central and western parts of the Netherlands. From the point that the Rhine-Meuse system became confluent with the Eridanos system, the specific petrographically controlled fingerprint of the Rhine deposits is lost, but remains inferable from the presence of Meuse flint in the gravel fraction. Contemporaneous deposits from Belgian rivers draining north and northeastwards (Stramproy Formation) in the southern part of the Netherlands, more specifically in the Roer Valley Graben, are also easily recognisable, but lose their specific petrographical characteristics as soon as they become confluent with the Rhine-Meuse fluvial system. However, the presence of easily recognisable Belgian river deposits at certain depths in the Roer Valley Graben proves that the Rhine did not continuously deposit sediments in the Roer Valley Graben.
Electron microprobe analysis of detrital garnets from Quarternary Rhine, Meuse and Baltic River sediments were studied by Tebbens et al. (Reference Tebbens, Kroonenberg and Van den Berg1995) to trace back the provenance and relative contributions from different source lithologies in each drainage basin. High-grade metamorphic almandine- and pyrope-rich garnets from the Vosges and Black Forest dominate the Rhine garnet suite in the Late Pliocene. With the onset of the Pleistocene, the Alpine Foreland Molasse is connected to the Rhine drainage area, supplying grossular- and probably also spessartine-rich garnets. The connection of the Aare and other Alpine tributaries to the Rhine in the Middle Pleistocene finally introduced large amounts of almandine-rich garnets derived from high-grade regionally metamorphosed inner Alpine source lithologies. The garnet suite of the Meuse sediments almost entirely consists of spessartines and Mn-rich almandines. They are derived from Mn-rich low-grade metamorphic pelites of the Libramont anticlinal region and the Stavelot Massif in the Ardennes. A small association of Mn-poor almandines is ascribed to a Vosges supply from before the capture of the Upper Meuse by the Moselle. The Baltic River garnet assemblages are characterised by a wide compositional spectrum, indicative of a large differentiated source area. The almandine- and pyrope-rich garnets are most likely derived from the extensive Fennoscandian Shield, while the spessartine-rich specimens are thought to originate from the mid-German Variscan massifs.
It is worth drawing attention to Krippner & Bahlburg (Reference Krippner and Bahlburg2013), who analysed detrital zircon on U–Pb ages and associated heavy mineral assemblages from Pleistocene Rhine River Middle Terrace sands and equivalents between the Swiss–German border and Cologne. They tested the assumed Alpine provenance of the sediments. According to these authors, the zircon age populations of all samples show similar distributions, their main age peaks being between 300 and 500 million years. Minor age populations are recognised at 570 and 1070 million years. Krippner & Bahlburg (Reference Krippner and Bahlburg2013) note the absence of ages younger than 200 million years and in particular any ages reflecting the Alpine orogeny between c. 100 and 35 million years in their age distribution of detrital zircon from Rhine River sediments. Therefore, these authors question the commonly made assumption of a direct delivery of detritus to the downstream Rhine River system from the Alps during at least some parts of the Pleistocene. Similarly, it would be necessary to reevaluate explanations on the provenance of Dutch sediments.
4.1.3 Modifications of the heavy mineral associations
The four principal factors that may modify the heavy mineral association from source to deposition are (1) weathering both in the source area and in the depositional basin, (2) mechanical abrasion during transport, (3) selective sorting of minerals according to size and density, and (4) chemical weathering after deposition. Size and density sorting in rivers generally leads only to small differences in the HMAs (Zonneveld, Reference Zonneveld1946; Van Andel, Reference Van Andel1950, Reference Van Andel1958; Kasse, Reference Kasse1988). Only where an erosion pavement has been formed by the winnowing out of the less dense grains do the heavier grains become concentrated, as happens during erosion of river bars and slopes. A low rate of sediment supply coupled with conditions of intense weathering can strongly modify the composition of the heavy mineral associations (Van Loon & Mange, Reference Van Loon, Mange, Mange and Wright2007; Van Loon, Reference Van Loon2009; section 5.1.6). During high rates of sediment supply (i.e. sediments generally derived directly from source areas where active erosion is occurring) the influence of all four factors is negligible and the heavy mineral assemblage directly reflects the petrography of the source area.
The impact of weathering is shown by a decrease in the less stable components pyroxene, amphibole and epidote, and a corresponding increase in the stable elements kyanite, staurolite, tourmaline, zircon and rutile (Table 5). In extreme cases this process can supposedly convert an amphibole-epidote association into a kyanite-staurolite-zircon assemblage (Weyl, Reference Weyl1949). Weathering of the heavy minerals in Dutch sediments has been described for podsol soil profiles (Van den Marel, Reference Van der Marel1949; Weyl, Reference Weyl1952a; Van Andel Reference Van Andel1952; Eisma, Reference Eisma1968) and silica sand deposits (Van Loon & Mange, Reference Van Loon, Mange, Mange and Wright2007; Van Loon, Reference Van Loon2009). In both cases the in situ weathering is caused by leaching of humic and/or other acids after deposition of the sediments. No quantitative data are given, but the podsol profiles have higher garnet fractions in the heavy minerals of their B horizons, indicating that minerals such as amphibole, epidote and saussurite are more easily dissolved. In general, soil formation in the Dutch fluvial sequences is weak and thus the effect of leaching is very small. The pedochemical weathering of the silica sands is described in section 5.1.6. Weathering of heavy minerals (as well as micas and feldspars) also impacts the groundwater composition by releasing cations such as Fe and Mg. This may be relevant for Dutch groundwater environments where Ca carbonate has been leached (Griffioen et al., Reference Griffioen, Vermooten and Janssen2013).
Table 5. Stability of some common heavy minerals under chemical weathering (Friis, Reference Friis1974).
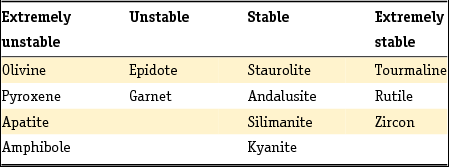
4.2 Clay minerals
4.2.1 Recent Rhine and Meuse clays
The clay mineralogy of Dutch fluvial clays has been studied by Breeuwsma (Reference Breeuwsma1985; Table 6), Adriaens (Reference Adriaens2014; Table 7) and also Favejee (Reference Favejee1951; Table 21). The latter performed the analyses only on air-dried samples, whereas Breeuwsma in addition also analysed probes of the samples treated with glycerol and probes treated with K and then heated to 300°C. Table 21 shows that using the method of Favejee, illite is the dominating clay mineral, with only minor amounts of kaolinite and smectite (montmorillonite). In contrast, the analyses of Breeuwsma (Table 6) show that the dominant clay minerals in the Rhine and Meuse are illite, smectite and vermiculite, with minor amounts of kaolinite and chlorite. Breeuwsma attributed these differences to the sample preparation, analytical technique and standard minerals Favejee used and confirmed the presence of vermiculite independently by the K-fixation capacity of the vermiculite bearing clays. Adriaens (Reference Adriaens2014) confirmed that illite and smectite are the major minerals in the Rhine and Meuse clays with minor amounts of kaolinite and chlorite, but he did not report vermiculite in the Rhine-Meuse clays studied. Using the same analytical method as Adriaens, Zeelmaekers (Reference Zeelmaekers2011) reported qualitiatively the possible presence of vermiculite in Rhine-Meuse sediments. The four data sets (derived from Adriaens, Zeelmaekers, Breeuwsma and Favejee) are not comparable and therefore conclusions on provenance or on the differences within fluvial clays or between fluvial and marine clays can be drawn from only one specific dataset. In this review we assume that the clay minerals are inherited from pre-existing parent rock or weathered material and have not been transformed or newly formed in the soils and sediments after deposition (Breeuwsma, Reference Breeuwsma1985; Tebbens et al., Reference Tebbens, Veldkamp and Kroonenberg1998). In the specific weathering studies reported, this is of course not the case.
Table 6. Mineralogical composition (in wt%) of the <2 µm fraction of some fluvial clays and marine clays in the Netherlands and muds from the North Sea surface (derived from Breeuwsma (Reference Breeuwsma1985), unless stated otherwise). Analyses were performed on samples after removal of organic matter, carbonates and sulphides.
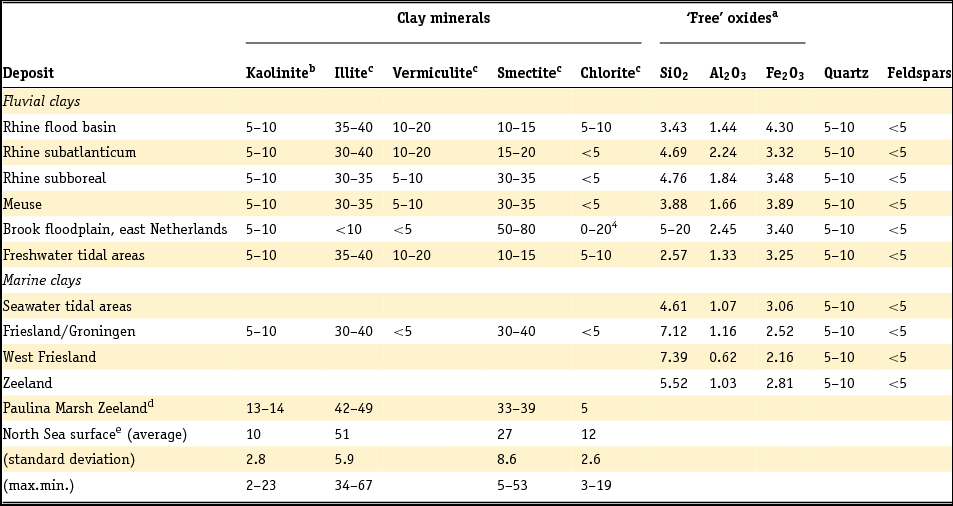
a Chemically determined: SiO2 and Al2O3 after boiling with 0.5 N NaOH and Fe2O3 after treatment with a dithionite-citrate-bicarbonate mixture.
b Chemically determined: after boiling with 0.5 N NaOH.
c The estimation is related to the total number of layers in clay minerals. Smectite and vermiculite occur only as components in mixed layer clays with illite.
d After Zeelmaekers (Reference Zeelmaekers2011).
e After Irion and Zöllmer (Reference Irion and Zöllmer1999), no details on whether humus, carbonates and sulphides were removed.
Table 7. Clay mineralogy (in wt%) of Rhine-Meuse-Scheldt Holocene and Pleistocene fluvial clays (derived from Adriaens (Reference Adriaens2014)).

The results reported by Breeuwsma (Reference Breeuwsma1985; Table 6) show that the main differences between the fluvial and marine clays are the vermiculite and smectite contents. Breeuwsma (Reference Breeuwsma1985) suggested that the absence of vermiculite in the marine clays is probably attributable to the presence of salt. Recent work by Skiba (Reference Skiba2013) shows that vermiculite selectively adsorbs K+ from seawater. Illitisation of dioctahedral vermiculite is very likely to occur in the early diagenesis of open systems in marine environments where sediments have unlimited contact with seawater. The chemical analysis of the <2 µm fraction shows further that there are also differences in the chemical composition: marine clays have higher iron contents. The K fixation and the CEC are greater in the fluvial clays than in the marine clays because the fluvial clays contain vermiculite and a smectite with a larger layer charge (Breeuwsma, Reference Breeuwsma1985). The vermiculite and smectite contents in clays from the Rhine differ, depending on whether the clay was deposited before or after Roman times (Table 6). Variation in smectite content in the Rhine clays is also documented by Adriaens (Reference Adriaens2014; Table 7); the total illite: total smectite ratio varies between below one (Rhine-Meuse-Scheldt Early Pleistocene), equal to one (Rhine-Meuse Mid-Holocene) and larger than one (Rhine Early Holocene; Scheldt Early and Mid-Holocene). Huisman and Kidden (Reference Huisman and Kiden1998) reported that for the lower units of the Waalre Formation (Early Pleistocene Rhine) the clay is dominated by well-crystallised smectite with illite and kaolinite whereas for the upper units it is dominated by illite and kaolinite. Such large variations in clay mineralogy may be attributable to drastic climate or provenance changes in time (Tebbens et al., Reference Tebbens, Veldkamp and Kroonenberg1998; Alizai et al., Reference Alizai, Hillier, Clift, Giosan and Hurst2012; Limmer et al., Reference Limmer, Kӧhler, Hiller, Moreton, Tabrez and Clift2012).
The alluvial brook clays from the eastern part of the Netherlands have extremely high smectite contents which are clearly higher than the smectite contents in the Rhine and Meuse. The smectites must originate from deposits present in the catchments of these brooks: possible candidates are Paleogene and/or Neogene marine deposits or Cretaceous carbonates (Breeuwsma, Reference Breeuwsma1985). Clays with such high smectite contents have very specific properties: high swelling capacities, CEC and K fixation.
4.2.2 Causes of changes in clay mineralogy
The Late Weichselian to Early Holocene transitional period (13–8 ka) was characterised by a general warming trend, interrupted by the Younger Dryas cooling phase. The effects of this climatic change on the composition of fine-grained residual channel infillings of the river Meuse were studied by Tebbens et al. (Reference Tebbens, Veldkamp and Kroonenberg1998). They demonstrated that although sediment provenance has not changed, residual channel infillings display a systematic bulk compositional change related to climate change. Late-glacial and Holocene climatic amelioration stabilised the landscape and facilitated prolonged and intense chemical weathering of phyllosilicates and clay minerals due to soil formation. Clay translocation and subsequent erosion of topsoils on Palaeozoic bedrock and Pleistocene and younger loess deposits increased the amount of smectite and vermiculite within river Meuse sediments. Smectite plus vermiculite contents rose from 30–40% in the Pleniglacial to 70–80% in the Holocene. The data presented by Tebbens and co-authors show that a steady-state supply is reached within 5000 years. Holocene sediments therefore contain higher amounts of clay that, compared to Late-glacial sediments, are richer in high-Al, low-K and low-Mg vermiculites and smectites. The above results illustrate that the composition of river Meuse sediments cannot be considered constant on a 1–10 ka timescale.
Huisman (Reference Huisman1998) reports a clear change in the clay mineralogy of the Brachterwald section (close to Venlo) of the fluvial Neogene Reuver Member (which is part of the Kieseloolite Formation). In the lower part of this section (0–5.75 m) the dominant clay mineral is kaolinite (kaolinite 53%, illite 25% and smectite 22%) whereas in the upper part the dominant clay mineral is smectite (kaolinite 34%, illite 18% and smectite 48%). This change is also documented in distinctly higher Ti/Al ratios in the section below 5.75 m and higher MgO content above 5.75 m. Boenigk (Reference Boenigk1974) found a shift in heavy minerals from local to Alpine provenance in the Brachterwald section, which appeared to be reflected by the above-mentioned drops in Ti/Al ratios and kaolinite contents and associated increase in smectite contents. Following Boenigk, Huisman (Reference Huisman1998) interpreted these changes as being caused by a shift from locally derived sediments (strongly weathered, with high kaolinite contents) to Alpine-derived sediments (less weathered and higher smectite contents).
4.3 Feldspars, micas and silica
Little attention has been paid to contents of other silicates such as quartz, feldspars and mica minerals in fluvial sediments. Quartz usually accounts for over 70% and is thus the dominant mineral in sands (Van Baren, Reference Van Baren1934). It is not the only SiO2 polymorph that is found in fluvial sediments: biogenic silica may be present as well. The information available on the concentration of biogenic silica in Dutch fluvial sediments and suspended matter is summarised in section 5. Van Baren (Reference Van Baren1934) classified the fluvial formations on their feldspar assemblage: the results are summarised in Table 4. The difference between sediments from the Eridanos and the Rhine river systems is noteworthy: the former are dominated by microcline and the latter by orthoclase and albite. Druif (Reference Druif1927) microscopically observed orthoclase, little or no microcline and even less plagioclase in a few samples from the Meuse and Gulp rivers. Muscovite was always abundantly present, whereas biotite was found in both Meuse samples and in one out of three samples from the Gulp river. Geologists have not systematically distinguished biotite from mica in mineralogical descriptions, which is unfortunate because the weathering rates differ considerably and biotite can be a source of Mg in pore water. Crommelin (Reference Crommelin1943) characterised the suspended matter of the Rhine for different grain size classes and compared it with Wadden Sea and North Sea sediments (Table 24). He found a relatively high content of micas in the riverine suspended matter.
The Na2O content of sediments has been used to characterise the Na-feldspar content, on the assumption that all Na is found in feldspars. This is probably true for sand or loam in Ca-HCO3 freshwater environments but unlikely for clay in an environment with Na-rich water such as seawater or fresh water having a Na-HCO3 composition. The latter is found when fresh water has displaced saline groundwater, as in the case of the fresh groundwater body around Hoorn. Under such conditions, the CEC will largely be occupied with Na and this will contribute to the Na2O-XRF content. Sediments have indeed been found to have a maximum Na2O content at an Al2O3 content of about 6–10% (Huisman, Reference Huisman1998, Reference Huisman1999), thereby establishing the occurrence of Na-feldspar in the silt and fine sand size class. Huisman & Kiden (Reference Huisman and Kiden1998) noted shifts in the Na2O content from less than 0.4% to 0.4–1.3% (i.e. less than 3.4% Na-feldspar to 3.4–11%) for Al2O3 content larger than 5% for fluvial sediments from the Tegelen and Kedichem Formations (currently classified as Waalre and Stramproy in the related study area). These shifts coincided with shifts in the heavy mineral assemblages from stable to unstable and were stated to be controlled by changes in the provenance of the Rhine from regional to Alpine as well as shifts between the Scheldt and Rhine systems. Similarly, Schokker et al. (Reference Schokker, Cleveringa, Murray, Wallinga and Westerhoff2005) studied the abundance of Na-feldspars in the Sterksel Formation from five sediment cores across the Roer Valley Graben and Huisman (Reference Huisman1999) made a more national inventory of fluvial formations. The Na2O content of about 1% is highest when Al2O3 is about 6–8%, coinciding with fine sand and not with loam, clay or coarse sand. Assuming all Na2O is present as Na-feldspar, the Na-feldspar then amounts to about 8.5%. Huisman (Reference Huisman1999) observed that the Late and Middle Pleistocene Kreftenheije, Urk and Sterksel Formations are richest in Na-feldspar, the Early Pleistocene Waalre and Stramproy intermediate, and the Neogene Kieseloolite and Early Pleistocene Scheemda (nowadays part of the Peize Formation) Formations poorest.
4.4 Ca carbonates
4.4.1 Carbonate contents
Fresh sediments of the River Waal (which is part of the Rhine system) deposited during normal flow conditions contain 12–20% CaCO3 where a weak relationship with the fine fraction is observed (De Groot, Reference De Groot1963). For high flow conditions, the carbonate content is about 12% and does not vary appreciably with the fine fraction. The carbonate content of recent Meuse sediment is lower: about 4% and invariant of the fine fraction. The carbonate content of suspended matter in the fluvial reaches of the Scheldt system is 11%, based on four sampling events (Zwolsman & Van Eck, Reference Zwolsman and Van Eck1999). The CaCO3 content of young sediments from Lake IJsselmeer varies only slightly with grain size from 0 to 75 µm: the content is about 10% (Verhoeven, Reference Verhoeven1962).
Table 8 presents statistical data on the carbonate contents of fluvial formations. The carbonate contents were determined by either the enrichment in total Ca content above a Ca/Al baseline (Bakker et al., Reference Bakker, Kiden, Schokker and Griffioen2007; Van Gaans et al., Reference Van Gaans, Hartog, Bakker, Kiden and Griffioen2007) or thermogravimetric analysis (other references mentioned in Table 8). Note that the contents determined by the former method are probably overestimated for clay and loam because the method does not take exchangeable Ca into account. On average, the clay and loam samples have higher contents than the sand samples for all formations, for reasons explained below. The high carbonate contents in sand of the Kreftenheije and Echteld Formations are striking, in particular in the Holland geotop region (which coincides with the provinces of North and South Holland, where marine deposits are present at or near the surface): the high contents in the Kreftenheije may be explained by local erosion and redeposition of Eem sand in this area in addition to accumulation of sediments from upstream (Busschers et al., Reference Busschers, Kasse, Van Balen, Vandenberghe, Cohen, Weerts, Wallinga, Johns, Cleveringa and Bunnik2007). Additionally, the Early and Middle Pleistocene fluvial formations that reach the surface were more prone to decalcification under palaeohydrological conditions (Griffioen et al., Reference Griffioen, Vermooten and Janssen2013). Table 9 presents an overview of the calcareous nature of a series of geological formations (Klein & Griffioen, Reference Klein and Griffioen2010), in which they combine a compilation of data obtained solely from thermogravimetric carbonate analyses and the description of the carbonate state of geological formations by De Mulder et al. (Reference De Mulder, Geluk, Ritsema, Westerhoff and Wong2003). The latter is based on qualitative HCl effervescence tests during borehole descriptions. One must realise that this test is indicative only and no effervescence happens when siderite or dolomite is involved. The analytical dataset mainly contains samples below the soil zone from the southern and northern Netherlands and is thus not representative at national scale. All fluvial formations are carbonate-poor to calcareous, although there are differences in range.
Table 8. Regional characteristics (as percentiles) of the pyrite, reactive Fe, organic matter and Ca carbonate contents in several Dutch fluvial geological formations.

d.l., detection limit, which is commonly around 0.1%.
The 82.5 percentile is given in regular and 90 percentile in italic.
M, Member; N-NL, northern Netherlands.
Table 9. General characterisation of the CaCO3 state of Dutch Quaternary geological formations (derived from Klein & Griffioen (Reference Klein and Griffioen2010)).

Different sedimentary facies of the Holocene confining layer (including the fluvial Echteld Formation) show different ranges in carbonate content (Table 10): crevasse-levee > flood basin clays > channel = loam bed > organic = aeolian inland dune (Van Helvoort et al., Reference Van Helvoort, Griffioen and Hartog2007). Here, the low carbonate content in the aeolian inland dunes is the result of decalcification during the Early Holocene when the groundwater table was considerably lower than during Late Holocene and carbonate leaching could be more intense (see also Pons, Reference Pons1957). Fragments of freshwater shells have typically been found in channel belt deposits. They also occur more sparsely in the heavy clayey flood basin deposits, where they are relics of the freshwater marsh fauna (Egberts, Reference Egberts1950).
Table 10. Median values of pyrite, organic matter and Ca carbonate contents (in wt%) in different facies of the Holocene confining layer in the Bommelerwaard polder (derived from Van Helvoort et al. (Reference Van Helvoort, Griffioen and Hartog2007)).
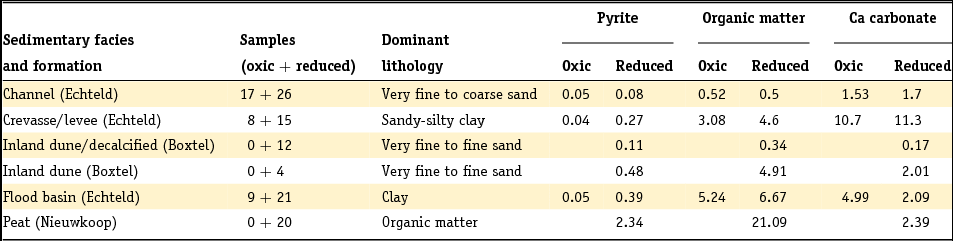
The calcareous silty sediments of the Wijchen Member (sandy clay overbank deposits and residual channel deposits; part of Kreftenheije Formation) with high percentages of the 20–80 μm fraction were found to contain higher CaO and MgO contents (Tebbens et al., Reference Tebbens, Veldkamp and Kroonenberg1998). Tebbens et al. attributed this to a loess admixture of these sediments, where the loess contains both calcite and dolomite.
Ca carbonate has been found to be related to the fine fraction (<16 μm) in Rhine deposits of the Holocene riverine area in the central Netherlands (Egberts, Reference Egberts1950; Pons, Reference Pons1957; Van Helvoort et al., Reference Van Helvoort, Filzmoser and Van Gaans2005). The highest contents (up to 8% CaCO3) were found in soils containing a fine fraction of 30–35%, predominantly in silty crevasse-levee deposits. The associated topsoils may have low contents due to decalcification (see later). Soils that contain a fine fraction (i.e. <16 μm) of about 60–65% do not contain CaCO3 above 0.5%. Results further point out that calcite is negligible or absent in the clay fraction (<2 μm) of fluvial clay deposits and also suspended matter (Van der Marel, Reference Van der Marel1950): less than 6% of total carbonate in the soil is present within the clay fraction.
A strong contrast exists in carbonate content between Rhine and Meuse sediments for Holocene fluvial deposits in the central-eastern Netherlands (Pons, Reference Pons1957; Hakstege et al., Reference Hakstege, Kroonenberg and Van Wijck1993). The Holocene deposits of the river Meuse are calcareous upstream from Venlo because of an influx of calcareous loess (Stiboka, 1972). They are carbonate-free or poor (<0.5%) downstream. This low carbonate content cannot always be attributed to decalcification during pedogenesis (Pons, Reference Pons1957): the suspended load of the Meuse has a CaCO3 content similar to that of the Rhine. The low CaCO3 content of channel belt deposits of the Meuse system is therefore attributed to the inflow of acid water into the Dutch stretch of the river Meuse and the associated enhanced dissolution of suspended carbonate. This acid water originated from the bogs of the Peel before these bogs were dug away for fuel in historical times, as well as from acid tributaries of the Meuse River. An alternative explanation is provided by Van Helvoort (Reference Van Helvoort2003), who proposed that the variability of the carbonate contents in channel and floodplain deposits is probably caused by the uptake of decalcified cover sands by Meuse-dominated channels and, in the case of the floodplain deposits, by acidifying oxidation reactions in the topsoil due to large-scale drainage in recent times.
4.4.2 The occurrence of dolomite
There is various evidence of dolomite occurring in fluvial sediments in addition to calcite. Using XRD, Salomons (Reference Salomons1975) characterised the mineralogy of recent freshwater sediments from the Biesbosch area sampled before closure by the Delta works and found that the dolomite fraction was about 15% of the total Ca carbonate content for lithological samples where 50% <20 μm, i.e. silt or clay samples. The ratio of acetic acid soluble carbonate to HCl soluble carbonate is close to 0.90 for the clay fraction of fluvial clay soils (Van der Marel, Reference Van der Marel1950) and varies by several per cent around 0.9% for the soil as a whole, which suggests about 10% Ca carbonate as dolomite in both cases. Similar fractions are deduced from Smittenberg et al. (Reference Smittenberg, Huisman, Veldkamp, Jongmans, Van Os and Huisman1998) for carbonate in the Tegelen Formation (now the Waalre Formation) in central Limburg, on the basis of XRF data. The allochthonous origin was further confirmed by observing detrital grains under a scanning electron microscope. For grain size classes >10 μm, Crommelin (Reference Crommelin1943) observed microscopically that after removal of calcite, per 100 grains of suspended material of the river Rhine there were zero to 4 grains of dolomite and zero or 1 grain of dolomite per 100 grains of suspended material of the Ems.
4.4.3 Carbonate isotopes
Fluviatile detrital Ca carbonates may originate from eroded ancient marine carbonates and soils. Salomons (Reference Salomons1975) characterised the δ13C and 14C age of Ca carbonates in recent sediments of the rivers Rhine, Meuse (sampled at the Biesbosch before closure) and Ems (at Diele; Table 11). Summarising, he found that the river sediments are influenced by the supply of pedogenic carbonate for two reasons. First, the 14C age of Rhine and Meuse carbonates was around 15 percent modern carbon (henceforth referred to as PMC) instead of 0% and that for the Ems was as much as 34 PMC. Second, the δ13C value is lighter than that for marine carbonates: below –3‰ instead of around 0‰. Calcite may also be formed authigenically under early diagenetic conditions within gyttja deposits, which are found in infillings of oxbow lakes (Smittenberg et al., Reference Smittenberg, Huisman, Veldkamp, Jongmans, Van Os and Huisman1998). Smittenberg et al. also described the occurrence of calcite as a cementing agent in deposits of the Waalre Formation, without providing an explanation. The δ13C value of Lake IJsselmeer sediments is unique in the sense that it is the result of a Holocene marine material mixing with recently precipitated carbonate. The latter is or was the result of supersaturation associated with algal blooms and increased pH (Salomons & Mook, Reference Salomons and Mook1980). The newly formed Ca carbonate has a δ13C value of +2.2‰ and the observed average composition of the sediment was +0.8‰, which differs by 3.4‰ from the value for the IJssel and Rhine rivers.
Table 11. Results of isotope analysis on carbonates in several Dutch geological sediments and the Belgian Boom Clay.

a Reported as inorganic C, which is impossible given the molar masses of CaCO3 and C.
The isotopic composition of carbonate in Pleistocene fluvial sediments varies (Table 11). Hartog et al. (Reference Hartog, Griffioen and Van Bergen2005) found three groups for the Kreftenheije Formation samples in eastern Gelderland: (1) samples having isotopic signature for both δ18O and δ13C, which is the same as that for present-day marine carbonates, i.e. carbonate probably of allochthonous origin, (2) samples depleted in δ18O, reflecting syngenetic carbonate formation during glacial periods and (3) samples depleted in both δ18O and δ13C reflecting post-depositional carbonate precipitation under groundwater influence. Unfortunately, the elemental composition of the various carbonate samples is unclear. The latter group is probably Fe-bearing carbonate such as siderite or ankerite. Carbonated sandstone can be found at the flanks of ice-pushed hills in central Netherlands, where a series of isotope analyses show depleted values in both δ18O and δ13C (Table 11; Post, Reference Post2006). These values are enriched relative to regional groundwater and the exact mechanism that explains this feature has not been clarified.
4.4.4 Decalcification
Decalcification of fluvial sediments occurs in different settings. First, it happens under pedogenesis at the local scale. Van den Berg & Loch (Reference Van den Berg and Loch2000) attributed decalcification of fluvial wetland soils in the Biesbosch to a combined effect of increased CO2 partial pressures during wet periods and pyrite oxidation with associated carbonate buffering in periods of aeration under fluctuating hydrological conditions. Van den Berg & Loch calculated a decalcification rate of about 3 mg CaCO3/(g soil.y) for the upper 20 cm of the soil, which must be considered as an upper limit, given the assumptions made. Pons (Reference Pons1957) characterised local decalcification during pedogenesis for different kinds of fluvial sediments. He distinguished different facies as well as age (before or after Roman times) and origin (Rhine or Meuse). The results are presented in Table 12. Jongmans and Miedema (Reference Jongmans and Miedema1986) studied the existence of small remnants of calcareous sediments in Late Weichselian Rhine sediments devoid of carbonate as found in former river channels in the eastern Netherlands. They explained these remnants as originating from slabs of frozen, calcareous loamy sediments, transported in floes and redeposited in sandy channel belts under periglacial conditions. These slabs have been less decalcified than the surrounding sandy sediments due to their poorer permeability in combination with preservation below the groundwater table and/or higher initial carbonate content.
Table 12. Overview of the original and present-day carbonate contents of soils derived from different fluvial sediment types of the Rhine and Meuse river systems in the eastern Netherlands (derived from Pons (Reference Pons1957)).

a Note that these river loam soils consist of clay, according to current Dutch lithological classifications.
The boundary between ‘poor’ and ‘rich’ carbonate content is not explicitly defined but ‘very poor’ is written to be less than 0.5%.
Second, regional decalcification of aquifers is a process at the geological time scale. For example, the decalcification of 1 m of sediment containing 1% CaCO3 requires 250 years when 80 mg Ca/l dissolves, porosity is 0.33 and recharge rate is 360 mm/y. Here, 80 mg/L can be considered as a typical Ca concentration in groundwater. Palaeohydrological decalcification at the regional scale was investigated by Hemel & Stuurman (Reference Hemel and Stuurman1999) for the Roer Valley Graben area, North Brabant. They compiled information on the carbonate state of geological deposits from borehole descriptions. They argue that, at the regional scale, all fluvial deposits in the study area were calcareous at the time of sedimentation. In the course of tens of thousands of years the Middle Pleistocene Sterksel Formation was decalcified at regional scale below infiltration areas but not completely towards the exfiltration areas (i.e. the brook valleys). As a result, the depth of decalcification varies considerably at the regional scale: sediment from drillings down to about 100 m depth can be essentially non-calcareous. The coarse sands of the Beegden Formation that were deposited on top of this formation in part of the area were also strongly decalcified. The young Kreftenheye Formation overlying these sands is still dominantly calcareous because of its limited age and hydrogeological position close to the present river Meuse, i.e. on the downstream side of groundwater systems. Holocene channel belt deposits along the ice-pushed hills of Nijmegen and Montferland have also become decalcified by exfiltration of acid, CO2-rich groundwater that is unsaturated for calcite (Pons, Reference Pons1957). This is a related mechanism acting at a shorter time scale and over smaller areas.
A third decalcification mechanism proposed by Smittenberg et al. (Reference Smittenberg, Huisman, Veldkamp, Jongmans, Van Os and Huisman1998) is calcite dissolution in conjunction with siderite precipitation under palaeohydrological conditions. One may wonder whether this recrystallisation occurs immediately below infiltrating areas or in parts of groundwater systems further downstream. The Fe(II)-rich and calcite undersaturated groundwater that is a prerequisite for this process is unlikely for groundwater infiltration areas under palaeohydrological conditions: Griffioen et al. (Reference Griffioen, Vermooten and Janssen2013) observed that the highest Fe concentrations in the southern Netherlands are related to high SO4 concentrations as result of enhanced atmospheric deposition and pyrite oxidation by extensive nitrate leaching. This applies to young groundwater under infiltration areas.
4.5 Reactive Fe minerals: sulphides, oxyhydroxides, carbonates and phosphates
4.5.1 Iron oxyhydroxides
Selective oxide extractions indicate the presence of Fe oxyhydroxides, where it must be remembered that other minerals may also dissolve to a certain extent in these extractants. Van den Berg et al. (Reference Van den Berg, Loch and Winkels1998) and Canavan and co-workers (Reference Canavan, Slomp, Jourabchi, Van Cappellen, Laverman and Van den Berg2006, Reference Canavan, Van Cappellen, Zwolsman, Van den Berg and Slomp2007) applied Fe oxide extractions to recent shallow sediments (<0.4 m) of the Biesbosch and Haringvliet, respectively (Table 13), where SO4 reduction was ongoing and thus anaerobic conditions prevailed. Using oxalate extraction, Van den Berg et al. (Reference Van den Berg, Loch and Winkels1998) measured 1–2% Fe2O3 as amorphous Fe hydroxide rather invariant with depth. Canavan et al. (Reference Canavan, Slomp, Jourabchi, Van Cappellen, Laverman and Van den Berg2006, Reference Canavan, Van Cappellen, Zwolsman, Van den Berg and Slomp2007) found about 0.5% ascorbate-extractable Fe2O3 and 0.8–2.2% citrate-dithionite-bicarbonate-extractable Fe2O3: the latter decreased more markedly within the depth profile. The presence of extractable Fe oxide at larger depth is attributed by Canavan et al. (Reference Canavan, Slomp, Jourabchi, Van Cappellen, Laverman and Van den Berg2006) to the partial unavailability of chemically reducible Fe(III) for microbial respiration. Thomas (Reference Thomas2007) found comparable average Fe oxide contents in recent, mostly sandy alluvial sediments of the Dommel and Dinkel brooks. The associated standard deviations were large, indicating a strong non-normal frequency distribution. The considerable difference in averages between the two brooks is noteworthy, as the grain size distributions were similar.
Table 13. Contents of extractable Fe oxyhydroxides and total Fe (as range or average with standard deviation) in recent sediments of fluviatile water systems.
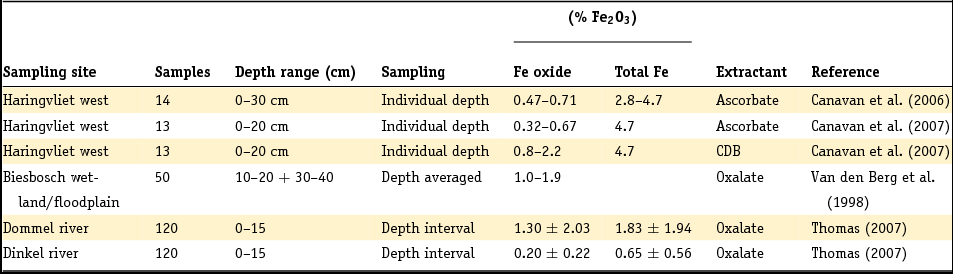
CDB, citrate-dithionite-bicarbonate extraction.
Selective Fe extractions were also applied by Buijs & Van der Grift (Reference Buijs and Van der Grift2001) to samples collected anaerobically at approximately 160 m depth from the Pliocene Kieseloolite Formation in Limburg. The weak hydroxylamine extraction gave on average somewhat lower contents than the strong oxalate extraction: 0.2–0.4 wt% Fe2O3 for the sand samples and 0.7–5.0 wt% for the clay samples. The total Fe2O3 content (3–25%) was also anomalously high in these clays, and respiration experiments revealed the presence of siderite. Secondary Fe enrichments were thus present in these clays. Two questions remain: whether true Fe oxides were extracted by the selective Fe oxide extractants, and how siderite interfered in these extractions.
A few studies have investigated Fe oxides in Dutch sediments in a direct, mineralogical manner. Riezebos (Reference Riezebos1971) and Riezebos et al. (Reference Riezebos, Bisdom and Boersma1978) isolated opaque and subopaque grains from samples from terrace deposits and Holocene sediments of the river Meuse in southern Limburg and identified them by microscopy. Riezebos (Reference Riezebos1971) observed that 90% of the opaque grains were goethite or lepidocrite in old Pleistocene and younger Pleistocene terrace deposits (Kosberg and Geertruidenberg levels, respectively). These grains have diameters in the sand class range. The Holocene Meuse deposits revealed a similar high content of goethite in the opaque fraction (Riezebos et al., Reference Riezebos, Bisdom and Boersma1978). Goethite was present as detrital grains. Rutile made up about 5% of the opaque grains and the remainder were ilmenite, leucoxene (finely crystalline rutile), magnetite and martite (pseudomorphic haematite). These minerals occurred as single grains and in rock fragments. Leucoxene and martite are weathering products under atmospheric conditions of ilmenite and magnetite, respectively. The Pliocene terrace deposits displayed a very different picture: the opaque minerals were dominated by rutile and leucoxene (70 and 20%, respectively), with ilmenite as minor mineral. This composition shift was explained as an enlargement of the drainage area of the river Meuse at the beginning of Quaternary (Riezebos, Reference Riezebos1971). Nelson & Niggli (Reference Nelson and Niggli1950) isolated opaque and subopaque grains from Meuse deposits on the high terrace in northern Limburg and performed XRD analysis on them after pulverisation. This sample comprised ilmenite and rutile as opaque minerals. A high detrital goethite content is thus characteristic of Quaternary Meuse sediments (if the effects of diagenesis on goethite can be excluded). Ilmenite and rutile can be secondary minerals, but their occurrence as individual grains as well as rock fragments indicates that they were formed prior to fluvial transportation. The leucoxenisation of ilmenite was found to be different for the two types of ilmenite, which makes it unlikely that this happened postdepositionally under the hydrological conditions present in Limburg.
Zhang et al. (Reference Zhang, Slomp, Broers, Bostick, Passier, Bottcher, Omoregie, Lloyd, Polya and Van Cappellen2012) used highly advanced spectroscopic techniques (sulphur K-edge X-ray near-edge structure and iron X-ray absorption spectroscopy) to characterise six Pliocene and Pleistocene fluvial samples from the Peel Horst (northern Limburg) according to reactive Fe minerals. As expected, the relative amount of Fe(III) oxides decreased with depth in favour of the amount of pyrite. Iron(II) oxides were substantially present in five out of six samples, which might be attributed to magnetite. Three samples contained near-similar amounts of Fe(III) oxides and pyrite, which were situated in the nitrate reduction zone. It cannot be assured that these minerals were mutually present under natural conditions or due to enhanced denitrification following intensive nitrate leaching. Thompson et al. (Reference Thompson, Cameron, Schwarz, Jensen, Maenhaut van Lemberge and Sha1992) performed a rare study on the magnetic properties of several cores from boreholes across the southern Northern Sea, one of which extended down to 220 m below the sea bed in Dutch territory. The measurements indicate the presence of magnetite as large grains in predominantly fluvial sediments and smaller grains probably of greigite or titanomagnetite in predominantly marine deltaic sediments. It is noteworthy that the magnetite had not diagenetically altered to Fe sulphides under buried, marine conditions in these sediments that were up to Pliocene age.
4.5.2 Rattle stones and iron oxyhydroxides
A particular occurrence of Fe oxides is as rattle stones in sandy or gravelly fluvial and periglacial deposits. Rattle stones are concretions composed of concentric laminae of different compositions, in which the core has become detached from the mantle. The cavity formed may be up to 50% volume. Van Loef (Reference Van Loef2000) studied their mineralogical and chemical composition in detail. Rattle stones were sampled near the Rhine and Meuse from pits in fluvial Sterksel and Kreftenheije deposits as well as periglacial Drente deposits, and were characterised using Mossbauer spectroscopy, XRD and instrumental neutron-activation analysis (INAA). Goethite was the predominant Fe oxyhydroxide in both core and mantle, and the crystallite size was in the nanometer range. Illite and vermiculite were the major clay minerals in the core and the Fe content varied from 2% to 15%. The Fe content of the mantle was about 50% and decreased outwards with decreasing crystallinity of goethite. Lepidocrocite was identified in samples from the Kreftenheije and Drente Formations but not in those from Sterksel: this was attributed to slow transformation of lepidocrocite to goethite, as the Sterksel deposits date from the Middle Pleistocene and the other two from Late Pleistocene. Joosten (Reference Joosten2004) reported that rattle stones from the Veluwe area are mainly composed of lepidocrite. The samples studied were collected from abandoned pits in the ice-pushed ridges, which suggests that they probably originate from the Middle Pleistocene Urk Formation. If this is true, it contradicts Van Loef's explanation of slow transformation (Van Loef, Reference Van Loef2000): he suggested that lepidocrite would not be found in rattle stones of Middle Pleistocene age.
The genesis of these rattle stones has been debated. Loope et al. (Reference Loope, Kettler, Weber, Hinrichs and Burgess2012) searched for analogues with concretions in Cretaceous sediments in Nebraska (USA). Van der Burg (Reference Van der Burg1969) assumed that rattle stones were formed due to oxidation of allochthonous siderite concretions that were deposited with the sand or gravel in which they are found. Oxygen penetrated into the concretion and oxidatively dissolved siderite, whereas the mobilised Fe(III) precipitated as Fe oxide on the inner side of the dense, outer rim. Dissolved CO2 is produced during this reaction, which enhances the solubility of siderite. Because of differences in density, when siderite is converted into Fe oxide there is a loss in volume, which would have resulted in the void within the concretion. Van Loef (Reference Van Loef2000) assumes that the genesis is related to wetting–drying cycles of small clay layers within sandy sediments in groundwater-fed areas. He suggests that dissolved Fe(II) became oxidised and Fe oxides precipitated as the sediment dried, and the clay layer also fractured too. During repeated wetting–drying cycles, the core detached itself from the mantle upon drying out. The latter interpretation provides no mechanism for preferential transport of water and dissolved Fe(II) from the sandy sediment to the clay lens during a dry period and the assumed thickening of the rind outwards would result in the cementing of surrounding sand instead of clay (Loope et al., Reference Loope, Kettler, Weber, Hinrichs and Burgess2012). A more plausible explanation for the high porosities of the pores is a recrystallisation process, not desiccation of clay. Indeed, siderite concretions have been found in sand and gravel along the Meuse river near Venlo (Scheres, Reference Scheres2007), while these concretions are also known to be present in the surrounding Waalre clay layer (Westerhoff, Reference Westerhoff2009; Vriens, Reference Vriens2011). Van der Burg's explanation thus seems more likely to us.
4.5.3 Iron sulphides
AVS has been analysed by extraction on shallow sediments from lakes within the river Rhine system (Van den Berg et al., Reference Van den Berg, Buykx, Van den Hoop, Van der Heijdt and Zwolsman2001; Van Griethuysen et al., Reference Van Griethuysen, Meijboom and Koelmans2003, Reference Van Griethuysen, Van Baren, Peeters and Koelmans2004; Canavan et al., Reference Canavan, Slomp, Jourabchi, Van Cappellen, Laverman and Van den Berg2006, Reference Canavan, Van Cappellen, Zwolsman, Van den Berg and Slomp2007), recent river sediments (Van den Berg et al., Reference Van den Berg, Loch, Van der Heijdt and Zwolsman1999; Poot et al., Reference Poot, Gillissen and Koelmans2007; Thomas, Reference Thomas2007), floodplains (Poot et al., Reference Poot, Gillissen and Koelmans2007) and temporarily inundated wetlands (Van den Berg et al., Reference Van den Berg, Loch and Winkels1998; Table 14). AVS extractions have been interpreted in terms of non-pyritic reduced sulphur in the references cited. However, this sulphur may be attributed to aqueous S species, mackinawite, greigite, fine-grained pyrite or a combination of these four (Rickard & Morse, Reference Rickard and Morse2005). The highest contents (up to 100 μmol S/g) were found in the western part of Haringvliet, where AVS amounts to almost 50% of total acid-soluble S. Contents up to 20 μmol S/g were found in the other studies, where AVS usually account for less than 25% of total acid-soluble S.
Table 14. Sulphur contents (as range or average with standard deviation) in different kinds of recent sediments in fluvial systems or small pools and lake.

a As pyrite-S.
Depth-specific sampling has revealed that the sulphide content at the sediment/water interface is often but not always zero. Differences in the AVS contents observed have been attributed to differences in the depositional rates of sediment with a higher organic carbon content (which is a substrate for SO4 reduction) and more reduced conditions in areas of low hydrodynamic energy. Initial SO4 concentration does not appear to be a controlling factor. The early diagenesis of sulphides is hard to explain quantitatively by geochemical transport modelling of the S chemistry; the same applies to explaining the AVS contents as part of the total, aqua regia-soluble S contents (Canavan et al., Reference Canavan, Slomp, Jourabchi, Van Cappellen, Laverman and Van den Berg2006, Reference Canavan, Van Cappellen, Zwolsman, Van den Berg and Slomp2007).
Pyrite contents in fluvial sediments have mostly been determined by total elemental analysis, where either all total S is assumed to be present as pyrite or it is calculated as the difference between total S and AVS, and AVS is assumed to represent non-pyritic, reduced S (see section 3.4). Table 14 presents AVS and total S data for recent fluvial sediments. Except for most of the samples of Thomas (Reference Thomas2007) and several riverine samples of Poot et al. (Reference Poot, Gillissen and Koelmans2007), the samples studied were clayey. The total S contents were usually several thousands of milligrams per kilogram and the AVS contents were much smaller. Following the assumptions made by the authors, the pyrite contents often vary between 0.1% and 1%, as 1000 mg S/kg is equivalent to 0.18% pyrite. A relevant question is how representative these values are for the pre-industrial period because human activities have caused S to become mobilised in the environment. It is worth noting that both the S content and the Fe oxide content (Tables 13 and 14) are lower in the Dinkel brook than in the Dommel brook and the Rhine river system. The presence of reactive Fe minerals thus varies regionally within the Dutch surface water systems.
Griffioen et al. (Reference Griffioen, Klein and Van Gaans2012) present cumulative frequency distributions per lithological class for shallow geological formations (<30 m depth) in the Roer Valley Graben and the Holland area that were mostly fluvial. Several observations are worth mentioning. First, these distributions are frequently concave, i.e. many samples with low content and a small group of samples with high contents. Second, the clay class has higher pyrite contents than the sand class, except for the Upper Pleistocene Kreftenheije Formation in Holland: the contents are commonly below 1% in sand and run up to 1–8% in clay. Third, statistically significant differences between the frequency distributions were found between the sand groups but not between the clay groups. Table 8 presents an overview of pyrite contents for several geological formations characterised in different regions following a similar approach to that of Griffioen et al. (Reference Griffioen, Klein and Van Gaans2012). Another general observation is that the maximum values are frequently three or more times higher than the 90 percentiles, which underlines the concave nature of the cumulative frequency distributions and the frequent existence of outliers. The sediment petrological origin of these outliers is not clear. It is worth pointing out that the pyrite contents of the Kreftenheije and Echteld Formations in the riverine area are about as high as those found in the western Holland area, where SO4-rich marine groundwater would have been more abundant during the Holocene. It seems that the presence of seawater-derived groundwater was not the limiting factor in pyrite diagenesis for these formations (Van Helvoort et al., Reference Van Helvoort, Filzmoser and Van Gaans2005, Reference Van Helvoort, Griffioen and Hartog2007).
By means of factor analysis on geochemical data of Holocene fluvial sediments from the riverine area, Van Helvoort et al. (Reference Van Helvoort, Filzmoser and Van Gaans2005) found that sulphur was associated with sedimentary organic matter content. Similar findings were reported by Heerdink & Griffioen (Reference Heerdink and Griffioen2012) and Vermooten et al. (Reference Vermooten, Griffioen, Heerdink and Visser2011) for various geological formations present within 30 m depth in the northern Netherlands and Zeeland. These regional observations are in line with the microscopic observations of Van den Berg et al. (Reference Van den Berg, Loch and Winkels1998) and Huisman (Reference Huisman1998) on the association between S and organic matter. Van Helvoort (Reference Van Helvoort2003) and Van Helvoort et al. (Reference Van Helvoort, Griffioen and Hartog2007) found that for different sedimentary facies within the Holocene fluvial sediments the pyrite content is not dictated solely by lithological class (Table 10). The pyrite content decreased in the following order for the reduced series of samples: peat > calcareous inland dune ≅ flood basin clay > crevasse/levee > decalcified inland dune ≅ channel. The oxidised samples had pyrite contents at or below detection limit. This shows that the major controlling factors on pyrite content are not depositional sorting but peat formation together with post-depositional diagenesis and leaching. The highest contents are also not necessarily related to the clay class.
Using an electron microprobe technique, Van den Berg et al. (Reference Van den Berg, Loch and Winkels1998) noted that in recent sediments sulphide precipitates had a 1:1 molar ratio for Fe to S, where all Fe sulphide was associated with the presence of vascular cell structures. Such precipitates may be related to the original presence of Fe oxide precipitates on the root surface plus the decomposition of proteins in reed roots associated with SO4 reduction. Huisman et al. (Reference Huisman, Klaver, Veldkamp and Van Os2000) and Huisman (Reference Huisman1998) found that pyrite occurs as framboids of 10–50 μm (Fig. 5), irregular concentrations up to 5 mm or nodules of 0.5–2 cm diameter in Late Pliocene and Early Pleistocene fluvial sediments: large amounts were concentrated in organic-rich clay layers (5–15% Corg) of a few tens of centimetres thick within the clay deposits. The pyrite was microscopically either associated with organic matter or occurred as loose or complete infillings in cracks. Based on the micromorphological presence of illuviated clay in these cracks, Huisman and co-authors proposed that these S-rich, fluvial deposits (up to 5% S) had been subjected to marine or brackish influence postdepositionally. This is palaeogeographically debatable for these samples from Limburg (Westerhoff, Reference Westerhoff2009). Rijksgeologische Dienst (RGD; 1989) also noted small framboidal pyrite in clay sublayers of shallow (<35 m) Pleistocene Meuse deposits and in clay and sand of Pliocene Kieseloolite deposits on the Peel Horst. No bigger aggregates were observed in either of these deposits.

Fig. 5. Example of pyrite framboids adhered to a sand grain (above) and the micromorphology of a single framboid (below).
Table 8 presents statistical data on the ‘reactive Fe other than pyrite’ contents of fluvial, stratigraphical formations. These amounts cannot be said to be equal to the Fe oxide contents because siderite, vivianite and possibly glauconite may also be involved. As expected, the contents are higher in clay and loam than in sand. The contents in clay and sand vary around several per cent, which is comparable to the Fe oxide contents in recent fluvial sediments (Table 13). The median values in sand are consistently below detection limit, which indicates there are negligible amounts of the related minerals in these geological units. High contents are found in the riverine area (Echteld and Kreftenheije Formations) and the Waalre Formation (particularly on the Kempen Plateau). The mineralogical speciation of this reactive Fe is still enigmatic for Dutch sediments. Occasional findings by Huisman and Kiden (Reference Huisman and Kiden1998) using XRD and scanning electron microscopy (SEM) indicated that Fe enrichments occur mostly as siderite in the Waalre Formation and as oxyhydroxides in the Pliocene Kieseloolite Formation. These findings apply to sediments buried below 20 m depth, where groundwater is probably Fe anoxic. We presume that S was limiting for more complete pyritisation of total reactive Fe in these buried fluvial sediments.
4.5.4 Iron carbonate
Traces of siderite have been reported in the silt and clay fraction of suspended material of the Rhine and also the German Ems and Belgium Scheldt Rivers (Crommelin, Reference Crommelin1943; Wartel, Reference Wartel1977). Siderite is regularly found in fluvial deposits under different sedimentological conditions, but the mineralogical matrix is always almost completely devoid of vivianite and pyrite. It occurs in three situations.
First, siderite is found in finely laminated clay and silt layers of the Waalre Formation (Huisman & Kiden, Reference Huisman and Kiden1998; Smittenberg et al., Reference Smittenberg, Huisman, Veldkamp, Jongmans, Van Os and Huisman1998; Westerhoff, Reference Westerhoff2009; Vriens, Reference Vriens2011; Fig. 6). It appears as: (1) coating of Ca-rich siderite on dolomite grains, (2) nodules of Ca-rich microcrystalline siderite, 2–40 μm in diameter, (3) sand to silt sized carbonate concretions with Ca, Mg and Fe in varying ratios (4) concretions of several decimetres in diameter and (5) a gel. Vriens (Reference Vriens2011) studied the large concretions in more detail using XRD and observed calcite, siderite and a carbonate solid solution with a chemical formula of Ca0.1Mg0.33Fe0.57CO3. The large concretions show variable Fe and Ca contents from the centre to the rim, but without a clear trend. This is opposite to what has been found for carbonate concretions in the marine Boom Clay containing ferroan calcite (De Craen et al., Reference De Craen, Swennen and Keppens1999a; Laenen & De Craen, Reference Laenen and De Craen2004). Smittenberg et al. (Reference Smittenberg, Huisman, Veldkamp, Jongmans, Van Os and Huisman1998) supposed that siderite was formed postdepositionally under waterlogged conditions, when carbonate originating from dissolution of calcite reacted with Fe(II) supplied by groundwater and/or weathering mica. Vriens (Reference Vriens2011) found for siderite rather depleted δ18O and δ13C values of –2 to –5‰ and –10‰, respectively, and for Ca carbonate only depleted δ13C values of –6 to –7‰ in the concretions, which implies that straightforward reprecipitation of Ca carbonate to siderite cannot explain the diagenesis of siderite.

Fig. 6. Whitish siderite-rich layer in clay of the Waalre Formation in the Maalbeek quarry (near Venlo, Limburg).
The second situation in which siderite is found is when it has formed under early diagenetic conditions in Late Pleistocene or Holocene gyttja deposits in association with vivianite and S (as pyrite or organically bound; Smittenberg et al., Reference Smittenberg, Huisman, Veldkamp, Jongmans, Van Os and Huisman1998). Such gyttja deposits can be found as infillings in oxbow lakes within a fluviatile environment. The siderite results from decay of organic matter under methanogenic, freshwater conditions, which supply the bicarbonate, with an influx of Fe(II) dissolved in groundwater. As mentioned earlier, calcite may also form here. The carbonate was found as subangular concentric nodules of calcite and siderite. XRD analysis confirmed that the nodules occurred as a mixture of calcite and siderite instead of single ankerite. The latter is a mixed carbonate that can form under low-grade metamorphic or hydrothermal conditions (Deer et al., Reference Deer, Howie and Zussmann1966).
Third, siderite seems to be dispersed in anoxic aquifer sediments (Hartog et al., Reference Hartog, Griffioen and Van der Weijden2002, Reference Hartog, Griffioen and Van Bergen2005). Its presence is inferred from CO2 to O2 ratios produced during incubation experiments on aquifer sediment samples as well as depleted δ13C values for carbonate present in aquifer sediments (Table 11). A series of samples from the fluvial Late Pleistocene Kreftenheije Formation as well as periglacial Drente Formation show δ18Ocarb values as low as –3‰ and δ13Ccarb values even as low as –8‰. Borehole descriptions in which siderite is visually observed also point to the widespread occurrence of siderite in Dutch aquifer sediments. Hemel & Stuurman (Reference Hemel and Stuurman1999) deduced that the upper 40 m of the Roer Valley Graben area are siderite-free, except occasionally in the vicinity of the eastern and western faults at 20–50 m depth. Below 40 m, siderite is found within the Roer Valley Graben and its occurrence corresponds with that of Ca carbonate. However, borehole data on siderite are less numerous than those on Ca carbonate.
4.5.5 Other reactive Fe minerals
Two studies using selective extraction, one with additional microprobe analysis, concluded that ferric Fe and PO4 can be bound in an almost fixed molar ratio of 1.2 to 1.5 as a mineral phase instead of PO4 being sorbed or co-precipitated with Fe hydroxide (Van Eck, Reference Van Eck1982a,b; Hyacinthe & Van Cappellen, Reference Hyacinthe and Van Cappellen2004). Earlier, Nriagu & Dell (Reference Nriagu and Dell1974) had pointed out that tinticite, Fe3(PO4)2(OH)3•3H2O, or cacoxenite, Fe4(PO4)3(OH)3•12H2O, might control PO4 solubility in freshwater environments. Van Eck (Reference Van Eck1982a,b) studied suspended matter in Haringvliet and Hollands Diep. Hyacinthe & Van Cappellen studied recent fresh estuarine sediment of the Scheldt in Belgium and recent brackish estuarine sediment of the Western Scheldt. They found Fe(III) PO4 aggregates typically several tens of microns in size for both sites, with Mn co-occurring as a minor cation with an Fe to Mn molar ratio of about 10. In both the fresh sediment (Hyacinthe & Van Cappellen, Reference Hyacinthe and Van Cappellen2004) and the suspended matter (Van Eck, Reference Van Eck1982a,b) all reactive Fe was bound to PO4, whereas in the brackish sediment only about half of the reactive Fe was bound, leaving some reactive Fe for other solid components.
The Weerdinge Member of the Appelscha Formation is the only Dutch geological unit of fluvial origin for which glauconite has been systematically observed in small amounts (www.dinoloket.nl). The formation predominantly contains coarse sand with some gravel.
4.5.6 Redox reactivity
Incubation experiments on Dutch sediment samples showed that pyrite and siderite, together with sedimentary organic matter, are important reductants for oxidants entering aquifer sediments via groundwater flow (Hartog et al., Reference Hartog, Griffioen and Van der Weijden2002, Reference Hartog, Griffioen and Van Bergen2005; Van Helvoort et al., Reference Van Helvoort, Griffioen and Hartog2007). The three reductants may react in parallel when the sediment is exposed to dissolved oxygen. The preferential oxidation during the experiments revealed that by comparison with sedimentary organic matter, the pyrite was more reactive but siderite reacted more sluggishly. Field studies have shown that pyrite oxidation leads to denitrification of nitrate-rich groundwater under farmland at the regional scale in the southern and eastern Pleistocene parts of the Netherlands (Van Beek & Hettinga, Reference Van Beek, Hettinga and Abriola1989; Griffioen & Keijzer, Reference Griffioen, Keijzer and Cidu2001; Visser et al., Reference Visser, Broers, Heerdink and Bierkens2009; Zhang et al., Reference Zhang, Slomp, Broers, Passier and Van Cappellen2009). The rate of depyritisation can be roughly estimated analogously to the rate of decalcification, using similar figures for porosity and recharge rate. It takes 153 years for the depyritisation of one cubic metre of sediment containing 0.1% FeS2 when the infiltrate contains 50 mg NO3/l (the international drinking water limit) and no oxygen is involved in the depyritisation. This rate implies that extensive nitrate leaching due to intensification of agriculture since World War II has not resulted in regional-scale depyritisation of Dutch groundwater aquifers. The redox cline within an aquifer from suboxic, pyrite-free to anoxic, pyrite-bearing may have shifted a few metres in the past 60 years but not tens of metres. Locally, Van Helvoort et al. (Reference Van Helvoort, Griffioen and Hartog2007) could also distinguish oxidised, pyrite-poor and reduced pyrite-bearing sedimentary facies within the Holocene confining layer in the riverine area. The pyrite-poor facies occurred mainly in the upper 1–1.5 m, suggesting that pyrite has leached in historical time, triggered by anthropogenic drainage, whereas previously pyrite had been produced in an early post-depositional stage. If the leaching occurs under more open conditions with respect to oxygen, due to artificial drainage, the rate of depyritisation will be higher for the same pyrite content than the rate presented above.
4.6 Solid organic matter
4.6.1 Organic matter contents
Table 15 presents summarising data on organic C contents in suspended matter and recent sediments from diverse fluvial systems. In 1995 there was an extremely high peak flow for the Rhine and Meuse rivers. Suspended matter and sediment deposited during the resulting floods contained around 6–11% organic C (Wolterbeek et al., Reference Wolterbeek, Verburg and Van Meerten1996; Koelmans, Reference Koelmans1998). Floodplain sediments or aquatic sediments in floodplain lakes have contents varying from close to detection limit to about 15% (Wolterbeek et al., Reference Wolterbeek, Verburg and Van Meerten1996; Van Griethuysen et al., Reference Van Griethuysen, Meijboom and Koelmans2003, Reference Van Griethuysen, Van Baren, Peeters and Koelmans2004; Vijver et al., Reference Vijver, Spijker, Vink and Posthuma2008). The organic carbon contents associated with present-day or young material from the large rivers are thus generally substantial. Wolterbeek et al. (Reference Wolterbeek, Verburg and Van Meerten1996) noted that for the Waal and Meuse river systems the organic C in the 1995 flood sediment was about twice that of the content in the topsoil below, and somewhat less than that of the Rhine river system. This suggests that not only the suspended matter content increases with increasing discharge (Asselman, Reference Asselman1999), but so does the organic C content. The aquatic sandy sediments in the brooks of the Dommel and Dinkel catchments have low organic C (Thomas, Reference Thomas2007).
Table 15. Organic carbon contents (as range or average with or without standard deviation) in recent sediments in fluvial systems or related floodplains.

Table 8 presents statistical data on the organic carbon content of various fluvial geological formations in different regions. The median values are often below or close to the detection limit (which is approximately 0.1%) except for clay in the Echteld Formation and clay and loam in the Tijnje Member of the Urk Formation. The high percentile values also become substantial in sand of the Echteld Formation. The Holocene Echteld Formation thus contains more sedimentary OM than the Pleistocene Formations studied. Whether this is an intrinsic characteristic of the sediments or a result of less prolonged OM degradation cannot be ascertained.
4.6.2 Carbon isotopes
The isotopic composition of particulate organic matter in recent sediments and suspended matter close to the mouths of the large rivers was studied by Salomons & Mook (Reference Salomons and Mook1981) and Laane et al. (Reference Laane, Turkstra, Mook, Ittekot, Kempe, Michaelis and Spitzy1990) (Table 16). The data show that the organic matter in the fluvial systems is predominantly land-derived material and old organic matter is present, for example 65 PMC is equivalent to a radiocarbon age of 3560 years. The suspended organic matter from the Rhine has somewhat lower δ13C values than the sedimentary organic matter. Middelburg and Herman (Reference Middelburg and Herman2007) found that by comparison, suspended organic matter from the Meuse had somewhat lower δ13C values; Hellings et al. (Reference Hellings, Dehairs, Tackx, Keppens and Baeyens1999) found seasonally fluctuating δ13C values between –32 and –27‰ for the upstream, fresh part of the Zeeschelde in Belgium. This suggests that more riverine algae or its detritus is present in the Meuse and the Scheldt than the Rhine. The δ13C values of sedimentary OM in a series of fluvial as well as marine and fluvioglacial deposits were determined by Hartog et al. (Reference Hartog, Griffioen and Van Bergen2005). They found an average of –25.1‰ ± 1.1 irrespective of geological formation or depth (Table 15). These values attest to the dominance of terrestrially derived OM.
Table 16. Observed ranges or averages with standard deviations in organic matter content and its δ13C and 14C values for suspended matter and recent sediments in fluvial, estuarine and marine systems.

F, Formation.
4.6.3 Organic geochemistry
Tambach et al. (Reference Tambach, Veld and Griffioen2009) characterised the bulk chemical properties of sedimentary organic matter in a small series of Early Holocene and Late Pleistocene fluvial sediments, using Rock–Eval pyrolysis. Here, the results for the unpurified samples will be summarised. The clastic samples from below the soil contained 55% to almost 80% refractory organic matter: the remainder was pyrolisable organic matter. For the latter, both the generation of hydrocarbons and CO/CO2 were recorded as a function of increasing pyrolysis temperature. This bulk characterisation illustrates that OM in aquifer sediment contains mostly refractory (inert) compounds.
More detailed molecular analysis of organic matter in suspended matter, recent sediments and geological deposits, all fluvial in nature, was performed by Van de Meent et al. (Reference Van de Meent, De Leeuw and Schenck1980, Reference Van de Meent, De Leeuw, Schenck and Salomons1985) and Hartog et al. (Reference Hartog, Griffioen and Van Bergen2005) using pyrolysis-MS with or without GC. Additionally, Hartog et al. (Reference Hartog, Van Bergen, De Leeuw and Griffioen2004, Reference Hartog, Griffioen and Van Bergen2005) studied Pleistocene glacial and Neogene marine deposits. It should be remembered that this method is basically semi-quantitative: an unresolved complex mixture of unidentified compounds is present and when samples are prepared using HCl/HF treatment, some OM is lost. Van de Meent et al. (Reference Van de Meent, De Leeuw and Schenck1980, Reference Van de Meent, De Leeuw, Schenck and Salomons1985) analysed samples from Lake IJsselmeer/Lake Ketelmeer and Haringvliet/Hollands Diep for the dominance of carbohydrates (including polysaccharides), proteins, lignin and peptides. The samples were taken in 1978, when algal blooms were abundant and eutrophication of these water bodies was much more severe than in the geological past or nowadays. For suspended matter they found a downstream increase in algae or bacterial biomass contribution that was probably the result of sedimentation of lignin-rich suspended OM in the upstream parts of these water bodies. They also found that OM in recent sediments contained more lignin compared to the OM in the suspended matter in the overlying water column. The difference is attributable to selective preservation of the refractory lignin-based compounds during settling and mineralisation. Similar findings were deduced from carbon isotopic analyses as described above.
A striking observation of Hartog et al. (Reference Hartog, Van Bergen, De Leeuw and Griffioen2004, Reference Hartog, Griffioen and Van Bergen2005) was that all fluvial, marine and glacial deposits were dominated by terrestrially derived OM, whereas in the marine Oosterhout samples no compounds of unequivocal marine origin were observed. This is in agreement with the stable carbon isotope analyses (Table 15). The interpretation was further based on four classes of compounds: (1) lignin-derived compounds (mainly guaiacyl based), (2) long-chain aliphatics, (3) fatty acids and (4) hopanoids. The presence of guaiacyl components, aromatics and long-chain alkanes with odd-over-even predominance reflects the importance of plant debris, while the absence of carbohydrate-based products indicates that degradation of the more labile substances of plant-derived OM (and also of phytoplankton) has occurred. The presence of guaiacyl compounds with or without preserved side-chains indicates the different intensity of oxidation among the samples: the fluvial Kreftenheije and periglacial Twente (now part of the Boxtel Formation) sand samples were almost depleted of guaiacyl components. No hopanoid-derived compounds were observed in these two samples either. A small input of bacterial hopanoids was observed in all other samples: this indicates the presence of dead bacterial biomass. Further, the odd-over-even predominance of the long-chain n-alkanes was most pronounced in these two samples, as was the aliphatic signal derived from macromolecular structures versus that from the ‘free’ low-molecular-weight compounds. Thus, although these samples were geologically the youngest studied, they exhibited the most advanced degradation of sedimentary OM. Hartog et al. (Reference Hartog, Van Bergen, De Leeuw and Griffioen2004, Reference Hartog, Griffioen and Van Bergen2005) therefore concluded that sediment age is in itself not the dominant factor controlling the degradation status of sedimentary OM. The difference between a clay sample and a sand sample (both from the Kreftenheije Formation, where the clay has a better preserved OM status), reveals that the source cannot be the dominant control. A major controlling factor seems to be the duration that sediments were exposed to oxygen.
5 Marine and estuarine sediments
The marine geological deposits comprise the Holocene Naaldwijk Formation, the Late Pleistocene Eem Formation, the Early Pleistocene Maassluis Formation and a continuous series of Palaeogene and Neogene formations (Fig. 4). They are widespread in the Netherlands and the adjacent Dutch part of the North Sea. During the Quaternary, they were deposited in relatively short periods in the onshore part of the Netherlands. The most western deposits of the Early Pleistocene Waalre Formation and the most northwestern deposits of the Middle Pleistocene Urk Formation are also estuarine, although these formations are basically fluvial deposits from the Rhine system. In addition to these buried deposits, recent sediments on the tidal flats and beaches have frequently been studied, as has suspended sediment, particularly in the Scheldt estuary. For the sake of completeness, the mineralogical literature on the Belgian part of the Scheldt estuary has been reviewed as well. Fig. 7 presents a map of the southern North Sea with all geographical names used in this paper.

Fig. 7. Map of the southern North Sea with the locations of the geographical names referred to in this paper.
5.1 Heavy minerals
5.1.1 Seabed sands in the southern North Sea
Baak's (Reference Baak1936) study of the heavy minerals of seabed sands in the southern North Sea was intended as a continuation and expansion of the pioneering studies by Edelman (Reference Edelman1933; see section 4.1). In total, Baak studied nearly 1000 samples and distinguished five HMAs (Table 17) that individually or as a mixture characterise the North Sea seabed sediments. For the distribution of the HMAs, see Fig. 8.
Table 17. Standard heavy mineral counts (in counts of 100) of the five HMAs distinguished by Baak (Reference Baak1936) in the bottom sands of the southern North Sea.
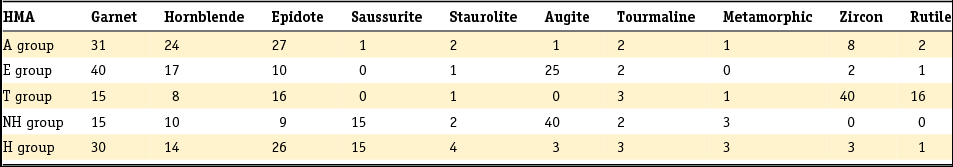

Fig. 8. Distribution of the heavy mineral assemblages across the southern North Sea (redrawn from Schüttenhelm & Laban, Reference Schüttenhelm and Laban2005).

Fig. 9. So-called ‘silver sand’ in the Beaujean quarry in southern Limburg.
The interpretations of Baak and later heavy mineral studies on the origin of the sands (Steinert, Reference Steinert1948; Eisma, Reference Eisma1968; Ludwig & Figge, Reference Ludwig and Figge1979; Henningsen, Reference Henningsen1987, Schwartz, Reference Schwartz1996) were compared by Schüttenhelm & Laban (Reference Schüttenhelm and Laban2005) with the results of recent seabed sediment mapping and sediment dynamics in the southern North Sea. Their findings for the five HMAs distinguished by Baak are summarised below:
• A group: with green hornblende, epidote and bright and colourless garnet. This group is identical to Edelman's A group (section 4.1). Geographically, this group is located along the German and Danish coast but also north and southeast of Dogger Bank and off Lowestoft (Fig. 8). All samples from deep boreholes in the German, Danish and Dutch sectors belong to this A association (Schwartz, Reference Schwartz1996). Baak interpreted this A group as having a northern Scandinavian provenance and as being formed during most of the Quaternary. This fits very well with the mapped fluvial and glacial units that were transported from Scandinavian source areas (Schüttenhelm & Laban, Reference Schüttenhelm and Laban2005).
• E group: with garnet and augite predominant and smaller amounts of hornblende and epidote. Hornblende is more frequent than epidote. The group is restricted to areas off the English northeast coast, south to the Norfolk Banks and the western Dogger Bank (Fig. 8). The E group, together with transitional associations, was interpreted by Baak to have a Scottish provenance on the basis of the association of peculiar garnet and augite. Admixed epidote and hornblende were thought to denote Scandinavian influxes and Baak suggested a Weichselian age. This group fits more or less with the distribution pattern of the various glacial units of Weichselian/Devensian age mapped during the Anglo-Dutch programme (Schüttenhelm & Laban, Reference Schüttenhelm and Laban2005).
• Tertiary (T) group: with zircon, garnet and rutile predominating, less epidote and hornblende, and with a high opaque percentage too. This T group occurs off the Suffolk and Essex coast and locally off the Northumberland coast and elsewhere. According to Baak the T group indicates the reworking of mature Tertiary and older sediments. Schüttenhelm & Laban (Reference Schüttenhelm and Laban2005) agreed with this conclusion and attributed the absence of the T group in the Belgian sector (Tertiary is present at a depth of 30 m below surface; Schwartz, Reference Schwartz1996) to the predominance of the younger H-type coastal sands.
• North Hinder (NH) group: with an augite, green hornblende and saussurite mineral assemblage, identical to Edelman's B-Lobith group (section 4.1). Here, the augite originates from the Rhine catchment. This group is found in the southernmost North Sea, away from the coast. This NH group must be related to the offshore low-stand Rhine deposits of Late Pleistocene age rich in green augite. However, the distribution of NH group samples and transitional samples is much larger than the mapped occurrence of the Late Pleistocene fluvial Kreftenheye Formation off the southwestern Dutch coast (Schüttenhelm & Laban, Reference Schüttenhelm and Laban2005). Recent work shows that in pre-Holocene times the ancestral Rhine must have extended far into the English Channel (e.g. Busschers et al., Reference Busschers, Kasse, Van Balen, Vandenberghe, Cohen, Weerts, Wallinga, Johns, Cleveringa and Bunnik2007; Toucanne et al., Reference Toucanne, Zararagosi, Bourillet, Gibbard, Eynaud, Giraudau, Turon, Cremer, Cortijo, Martinez and Rossignol2009). Large-scale, northward sand transport must have occurred during the initial Holocene transgression to get the geographical distribution of the NH group in line with the former river system (Van der Molen & Van Dijck, Reference Van der Molen and Van Dijck2000; Schüttenhelm & Laban, Reference Schüttenhelm and Laban2005).
• H group: with a garnet, epidote, saussurite and hornblende HMA, already considered by Edelman (Reference Edelman1933) to be a mixture of the HMAs he found along the Dutch coast. These reworked sands are omnipresent along the coasts of Dover Strait, western Netherlands and the Frisian islands. The saussurite percentages decrease to the north and east. From its distribution, Schüttenhelm & Laban (Reference Schüttenhelm and Laban2005) concluded that the various H group occurrences cannot be the result of one and the same mixing process. The samples in the English Channel result from alternating marine and fluvial sedimentation and related reworking. The highly dynamic Dover Strait area accounts for the highly mixed sands sampled there (Van der Molen & Van Dijck, Reference Van der Molen and Van Dijck2000). Over time, tidal amplitudes and currents continued to increase but the orientation of currents and the resulting sand transport in the eastern part of the Southern Bight gradually changed from perpendicular to parallel with the shore, which accounts for the presence of the H group sediments all along the Belgian and Dutch coast. The initial inlet coast type certainly aided the mixing process and brought H group sediments within the present coastline of the Netherlands too (Beets & Van der Spek, Reference Beets and Van der Spek2000). According to Baak (Reference Baak1936), but based on two samples only, the mixed provenance H group extends to the East Frisian islands. However, Ludwig & Figge (Reference Ludwig and Figge1979) found only A group assemblages (cf. section 4.1) on the shoreface and suggested that ongoing reworking of glacial deposits (Hoselmann & Streif, Reference Hoselmann, Streif and Streif2004) is reinforcing the existing A group and diluting any H group influxes brought in by the prevailing westerly longshore drift.
5.1.2 The Wadden Sea and the Scheldt estuary
The heavy minerals of the sands in the Wadden Sea and the Scheldt estuary were studied by Baak (Reference Baak1936) and in more detail by Crommelin (Reference Crommelin1940). They predominantly consist of garnet, epidote, hornblende and zircon, with minor quantities of saussurite, staurolite, tourmaline and other minerals (mix of the A group of Edelman with slight admixtures from Baak's H group). The fine sands along the mainland coast are comparatively rich in opaque minerals, epidote and zircon; the coarser sands in the north contain more garnet and a little more hornblende. The same compositional dependency on the grain sizes was observed for the Scheldt estuary (Van Straaten, Reference Van Straaten1954) but, as discussed by Van Straaten (Reference Van Straaten1954), the character of these relationships is nevertheless only of local significance. The composition of the Wadden Sea sands is very similar to that of the adjacent parts of the North Sea floor (cf. Baak, Reference Baak1936). The material consists of locally reworked Pleistocene material selected, transported and imported into the Wadden Sea by marine processes (e.g. Baak, Reference Baak1936; Crommelin, Reference Crommelin1940; Winkelmolen & Veenstra, Reference Winkelmolen and Veenstra1974, Reference Winkelmolen and Veenstra1980; Mulder, Reference Mulder2000; Hoselmann & Streif, Reference Hoselmann, Streif and Streif2004). The same conclusion was reached with regard to the sands in the estuaries of the provinces of North and South Holland and Zeeland (Baak, Reference Baak1936; van Veen, Reference Van Veen1936; Crommelin, Reference Crommelin1951). A small amount of sand and a larger amount of fines are imported with the currents flowing from the south parallel to the coast along the Dutch part of the Wadden (Schüttenhelm & Laban, Reference Schüttenhelm and Laban2005; Oost et al., Reference Oost, Hoekstra, Wiersma, Flemming, Lammerts, Pejrup, Hofstede, Van der Valk, Kiden, Bartholdy, Van der Berg, Vos, De Vries and Wang2012). Crommelin's and Baak's conclusions for the Wadden Sea were confirmed in a recent study (Oost et al., Reference Oost, Hoekstra, Wiersma, Flemming, Lammerts, Pejrup, Hofstede, Van der Valk, Kiden, Bartholdy, Van der Berg, Vos, De Vries and Wang2012) on the development of the barrier islands of the Wadden Sea. All the above-mentioned studies conclude that rivers have never brought substantial amounts of sediments into the Wadden Sea. Crommelin (Reference Crommelin1943) also studied the mineral composition of the silt fraction and came to the same conclusion: like the sand fractions, most of the silt fractions originate from the North Sea. Direct fluvial supply can have been of only subordinate importance. This applies both to the Wadden Sea silts and to those of the Scheldt estuary.
5.1.3 Beach and dune sands
Edelman (Reference Edelman1933) and Baak (Reference Baak1936) studied the HMAs present in the beach and dune sands along the Dutch west coast. Edelman's data are given in Table 18. A comparison with Table 17 shows that these sands belong to Baak's H group. The average mineral contents of the 200–250 µm fraction given by Eisma (Reference Eisma1968) differ considerably from those of the >30 µm fraction given by Edelman (Table 18). The Dutch coastal sands, i.e. dune, beach and shoreface sands, vary greatly in grain size and composition, but two main types of sand are distinguished:
1. High quartz (often coloured) contents (> 95%), with garnet as the dominant heavy mineral (Table 18). This type is found chiefly north of Bergen (beach pole 37) on the North Holland coast (Edelman, Reference Edelman1933; Baak, Reference Baak1936). These sands on the beaches and in the dunes are low in Ca and Mg (Eisma, Reference Eisma1968).
2. Lower quartz (less coloured) contents (<90%), with saussurite and hornblende dominating its HMAs (Table 18). These sands on the beaches and in the dunes have high Ca and Mg contents between km poles 44 and 92, increasing gradually between km poles 34 and 44 (Eisma, Reference Eisma1968).
Table 18. Average heavy mineral contents (in counts of 100) of the beach and dune sands deposited along the Dutch coast from Den Helder to Hoek van Holland. Data for the 200–250 µm fraction from Eisma (Reference Eisma1968) and for the >30 µm fraction from Edelman (Reference Edelman1933).

According to Edelman's data the boundary between the two types is sharp: mixing occurs over a distance less than 1 km. The differences in HMAs (Table 18) suggest a difference in origin. Edelman (Reference Edelman1933) found HMAs of the following sources in the coastal sands:
• River sands from the Rhine, Meuse and perhaps Scheldt. However, the recent supply of river sands of the Rhine, Meuse and Scheldt is very small (Van Veen, Reference Van Veen1936; Terwindt et al., Reference Terwindt, De Jong and Van Der Wilk1963) but conditions may have been different during the older, Holocene periods (Van Straaten, Reference Van Straaten1963).
• River sands from the Eridanos system.
• Sands present in the Saalian glaciation.
• Reworked Tertiary sands from the mouth of the Scheldt (which are easily recognisable from the accompanying Tertiary Bryozoa).
Eisma (Reference Eisma1968) used the same preparation and petrological method as Edelman (Reference Edelman1933) and Baak (Reference Baak1936), except that he studied four grain size classes between 100 µm and 315 µm separately. Only data for the 200–250 µm fraction are given in Eisma (Reference Eisma1968). Eisma found that north of the coastal village of Bergen (beach pole 37) both the beach sands and the fine fractions of the dune sands contain more heavy minerals than south of Bergen (km pole 37; Table 19). An identical geographical separation was found for the feldspar, Al, Ca carbonate, Mg carbonate and Fe contents, with the Al proportional to the feldspar content in these sands (Eisma, Reference Eisma1968). Table 19 also shows that the heavy mineral contents are not equally distributed over the separated fractions. The heavy mineral content of the finest fraction (100–160 µm) is 10 to 100 times more than that of the 200–250 µm fraction. Eisma reported that in the 200–250 µm and 250–315 µm fractions near beach pole 37 there is a sharp increase in the garnet and an even sharper increase in tourmaline, but a decrease in saussurite contents (higher to the north) (Table 19). However, this change is more gradual in the 160–200 µm fraction while in the 100–160 µm fraction no change is apparent.
Table 19. Heavy mineral content (in %) of individual size fractions of beach and dune sands collected between Scheveningen (km pole 91) and Den Helder (km pole 2) (derived from Eisma (Reference Eisma1968)).
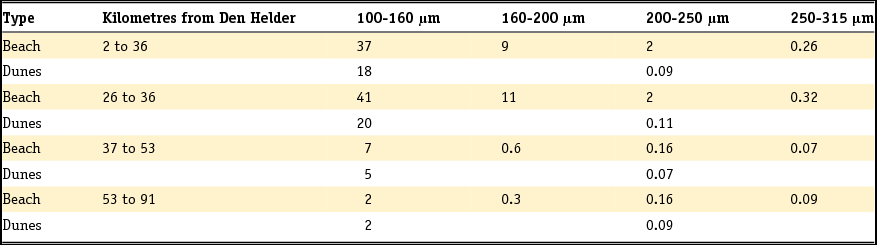
During the Holocene, the area north of Bergen remained land or was a shallow tidal flat separated by the so-called Bergen tidal inlet from the coastal sands further south. The Bergen tidal inlet was the largest tidal inlet in the Dutch coast and was closed around 3300 BP (Beets & Van der Spek, Reference Beets and Van der Spek2000). This northwestern headland with its core of glacial sands was gradually eroded, providing A group material. The transition in the HMAs along the Dutch coastal sands coincides with the Bergen tidal inlet, indicating that this inlet acted as an effective trap for northbound sands as long as it existed.
Eisma (Reference Eisma1968) attributed the co-occurrence of the two completely different HMAs at Bergen partly to the geologically recent, Holocene conditions along the coast and partly to the general history of the sands. His study confirmed the mixed nature of Baak's H group and the mixed provenance of the coastal sands since Saalian times. His interpretation was that the sands north of Bergen consist mainly of reworked glacial deposits from Saalian age and Rhine-Meuse sands having the S association (section 4.1). North of Bergen no carbonate is present in the beach sands and therefore their pH would have been low, which may have enhanced silicate weathering. This more severe weathering resulted in loss of some hornblende and probably also of augite, but its effect on the composition of the HMA was small (Eisma, Reference Eisma1968). The HMAs south of Bergen were interpreted by Eisma as reworked Rhine sands of the S association with an admixture of Late Pleistocene and Holocene Rhine sands of the AS association (section 4.1). The mixing occurred during the Eemian (by the sea), Weichselian (by the wind) and the Holocene (during coastal erosion and the formation of inlets). Erosion did not reach great depths except locally in the tidal inlets, which explains the virtual absence of an admixture of sands of the Eridanos system, usually present at a depth of some 25–60 m or less below the Saalian deposits.
5.1.4 Heavy mineral contents
The amount of heavy minerals has generally not been determined. Normally, it is less than 1–2% in the sand fraction of Dutch sediments (Druif, Reference Druif1927; Van Andel, Reference Van Andel1950; Wartel, Reference Wartel1977). Based on non-invasive radiometric seafloor mapping, De Meijer (Reference De Meijer1998) indicates that it may amount to up to a few per cent at the seafloor immediately north of Terschelling and Ameland. However, thin layers of beach sand have been observed that have several tens of per cent of heavy minerals (Crommelin & Slotboom, Reference Crommelin and Slotboom1945; Schuiling et al., Reference Schuiling, De Meijer, Riezebos and Scholten1985). These layers are referred to as garnet sand layers because garnet is the dominant heavy mineral and is responsible for the purple reddish or olive-green brownish colour. They are found along the entire Dutch coast from Zeeland to Ameland (Van der Sleen, Reference Van der Sleen1912; Crommelin & Slotboom, Reference Crommelin and Slotboom1945; De Meijer et al., Reference De Meijer, Lesscher, Schuiling and Elburg1990). Their origin is explained as storm erosion of dunes with a collapse on the beach and subsequent washing away of light minerals, leaving the heavy minerals. The sharp lower boundary of such layers fits this explanation. Crommelin & Slotboom (Reference Crommelin and Slotboom1945) found that such a layer consisted of 57% garnet and 18% opaque Fe oxides. De Meijer et al. (Reference De Meijer, Lesscher, Schuiling and Elburg1990) found up to 70% heavy minerals in a series of 16 samples but did not elucidate what kinds of layers were sampled at beaches along the coast.
5.1.5 Quaternary and Neogene marine formations
Investigations of Young Tertiary deposits in the Netherlands, Schleswig-Holstein and Denmark have shown an evident connection between depositional environment and heavy mineral associations of the sediments for sediments originating from Scandinavia. It seems to be a fundamental rule that such marine sediments are characterised by the unstable heavy minerals amphibole and epidote, whereas non-marine sediments are generally characterised by more stable heavy minerals and typically do not contain amphibole (Edelman, Reference Edelman1938; Weyl, Reference Weyl1950, Reference Weyl1952a,b, Reference Weyl1953; Larsen & Dinesen, Reference Larsen and Dinesen1959; Larsen & Friis, Reference Larsen and Friis1973). Table 20 illustrates that all the Dutch marine geological formations are characterised by unstable HMAs.
Table 20. Heavy mineral assemblages and glauconite presence of the Quaternary and Neogene marine formations. Data are from Westerhoff (Reference Westerhoff2009), Doppert et al. (Reference Doppert, Ruegg, Van Staalduinen, Zagwijn, Zandstra, Zagwijn and Van Staalduinen1975) and Baak (Reference Baak1936). No systematic data could be found on the quantities of feldspars in the marine geological formations.

5.1.6 Weathering and silica sands
Characteristic examples of bright white sands consisting almost exclusively of quartz (called ‘silver sand’ in the Netherlands) occur in the marine Breda Formation (Heksenberg Member; De Mulder et al., Reference De Mulder, Geluk, Ritsema, Westerhoff and Wong2003). They are well-sorted, fine-grained, with well-rounded grains and are considered to be beach or coastal sands (De Mulder et al., Reference De Mulder, Geluk, Ritsema, Westerhoff and Wong2003). The quartz content of this silica sand is at least 98% and the heavy mineral content is exceptionally low (0.003%; Van der Meulen et al., Reference Van der Meulen, Westerhoff, Menkovic, Gruijters, Dubelaar and Maljers2009; Van Loon, Reference Van Loon2009). The silica sand is overlain by a lignite unit, although in places this lignite has been entirely eroded. Van Loon (Reference Van Loon2009) hypothesised that the extreme weathering was most probably caused by infiltrating soil water rich in humic acids and by prolonged surficial exposure to weathering in a humid tropical or subtropical climate. Here, Van Loon (Reference Van Loon2009) also states that neither of these two options is completely satisfactory in itself to explain all weathering features of the silica sands: why is the lower boundary sometimes so sharp and where is the leached-out material? As well as containing very low proportions of heavy minerals, the sands also contain a limited variety of heavy minerals as a result of dissolution of the less stable minerals. The silica sand contains only three heavy minerals that are available for a weathering analysis: garnet, staurolite and tourmaline (Van Loon & Mange, Reference Van Loon, Mange, Mange and Wright2007). Tourmaline can be surprisingly strongly weathered in this silica sand, probably because of its specific chemistry (Van Loon & Mange, Reference Van Loon, Mange, Mange and Wright2007). Staurolite, when present, appears to be most suitable for assessing the intensity of weathering in the extremely weathered silica sand. One other surprising outcome is the co-occurrence of fresh and strongly weathered grains of heavy minerals such as garnet and staurolite, which are considered to have low resistance to weathering. To explain this, the term ‘differential weathering’ was introduced and this should have taken place at a small spatial scale (Van Loon, Reference Van Loon2009). The fragile weathered form of the heavily corroded grains indicates that they must have been produced in situ because they would have been destroyed by the vigorous action of waves and currents in their depositional environment.
5.2 Clay minerals
With the exception of the studies by Zuther et al. (Reference Zuther, Brockamp and Clauer2000), Pache et al. (Reference Pache, Brockamp and Clauer2008), Zeelmaekers (Reference Zeelmaekers2011), Koenen & Griffioen (Reference Koenen and Griffioen2013) and Adriaens (Reference Adriaens2014), who state that their results are quantitative or at least semi-quantitative, all the other studies cited below qualify their data at best as semi-quantitative. To understand the distribution of the clay assemblages in the North Sea, it is now generally agreed that clays in seawater are transported in flocs with a size of about 40 µm (e.g. Zeelmaekers, Reference Zeelmaekers2011).
5.2.1 Coastal sediments
The first systematic study of the clay mineralogy in the Netherlands was initiated in 1935 to solve the origin of sedimentary mud in the land reclamation of the Wadden Sea. As already discussed in the paragraph on the heavy minerals, not only the fine fraction was studied but also the silt and sand fractions (Crommelin, Reference Crommelin1940, Reference Crommelin1943). As the ‘Wadden’ mud may be composed of clastic material originating from the North Sea or the rivers Ems, Weser, Elbe and Rhine, various samples of these possible sources were investigated, together with mud from old clay banks and mussel banks in the Wadden Sea. Three fractions were studied after the samples had been treated with HCl and H2O2 to remove the organic matter, carbonate and sulphides (Favejee, Reference Favejee1951).
A compilation of the results is given in Table 21, in which the fluvial clays studied are also included. The figures show that the size fraction has a great influence on the mineralogy. The bulk of the clay fraction is made up of quartz and illite, with the first decreasing and the latter increasing in frequency with diminishing grain size (Table 21). The remainder is composed of kaolinite, montmorillonite, illite + muscovite and feldspars.
Table 21. Mineralogical composition (in wt%) of the fractions <0.5, 0.5–2 and 2–10 µm of mud deposited along the Dutch and German coast (derived from Favejee (Reference Favejee1951)).

I Recent Wadden mud of land reclamations along the coast of Groningen province.
II Mud from bottom sediments in the Wadden Sea: a, mussel banks; b, mud with shell fragments; c, mussel pellets.
III Old clay banks in the Wadden Sea.
IV Mud from suspended matter taken during ebb tide, south of Schiermonnikoog.
V Mud from suspended matter sampled in the tidal inlets of the Wadden Sea.
VI Mud from bottom sediments of the North Sea, south and southeast of the Dogger Bank and the German Bight.
VII Mud from marshes in Zeeland province.
VIII Mud in suspended matter from the rivers: a, Ems; b, Weser; c, Elbe; d, Rhine.
No significant distinction in mineral composition can be made between the muds from the Wadden Sea, from the Eastern and Western Scheldt estuaries, the North Sea floor and the suspended material in the tidal inlets (Favejee, Reference Favejee1951): the composition seems to be highly uniform along the whole Dutch coast. However, there is a noticeable difference between the marine muds and fluvial clays. The latter have a low content of montmorillonite and strongly varying percentages of kaolinite and quartz (Table 21; Favejee, Reference Favejee1951). The outcomes of the clay mineralogy (Favejee, Reference Favejee1951) and heavy mineral studies on the silt and sand fractions (Crommelin, Reference Crommelin1940, Reference Crommelin1943) in the Wadden Sea and the estuaries in Zeeland are similar: the sediments in the Wadden Sea and the estuaries in Zeeland are predominantly marine in origin (see also Van Straaten, Reference Van Straaten1954).
No further systematic studies have been performed, but Breeuwsma (Reference Breeuwsma1985) summarised the results of a number of small projects in which the clay minerals were determined and compared the characteristics of marine and fluvial clays. The results for the river and the marine clays are given in Table 6. The results show that the <2 µm fraction of Dutch soil clays comprises approximately 85% of clay minerals and the rest is made up of quartz, feldspars and oxides of Si, Al and Fe. Breeuwsma analysed samples from West Friesland and Zeeland but gives no semi-quantitative results for these areas in his paper. In the rest of this section we assume, in accordance with the findings of Favejee (Reference Favejee1951) and as discussed by Breeuwsma (Reference Breeuwsma1985), that the West Friesland/Zeeland and Friesland/Groningen clays have the same clay mineralogy. As pointed out in section 4.2, the major differences between recent marine and fluvial clays in the Netherlands are the differences in the vermiculite and smectite contents (Table 6). Analyses of the smectite-illite minerals showed that the Fe content in marine clays is much higher than in the fluvial clays and that because of the higher vermiculite content of the fluvial clays, the K fixation and CEC of the fluvial clays are higher (Breeuwsma, Reference Breeuwsma1985).
Transport studies of marine sediments along the Dutch coast (Terwindt, Reference Terwindt1977; De Groot, Reference De Groot1963; Eisma, Reference Eisma1981) all show that the amount of mud originating from the English Channel exceeds the discharge of fine material by the Rhine and Meuse. This conclusion is in agreement with the conclusions of Salomons (Reference Salomons1975) and Salomons et al. (Reference Salomons, Hofman, Boelens and Mook1975), who on the basis of the isotopic composition of both the carbonate and clay minerals estimated that no more than 20% of the mud in the Wadden Sea originated from the Rhine-Meuse delta. Based on the above literature, the difference in recent marine and fluvial clays (Rhine-Meuse delta) was attributed by Breeuwsma (Reference Breeuwsma1985) to a large southern North Sea component (erosion of English cliffs) in the marine clays.
In a thematically comparable study in which more advanced XRD analysis techniques were used, Zeelmaekers (Reference Zeelmaekers2011) compared the results of the Scheldt estuary with bottom samples from mudflats along the Belgian coastal zone and recent sediments from the Paulina salt marsh located about 20 km from the mouth of the Scheldt estuary (Tables 6 and 22). The age of the latter sediments was estimated to be between 50 and 100 years. For the marine part of the Scheldt estuary (including the Paulina salt marsh) the clay mineralogy was found to be identical with that of the mudflats along the Belgian coastal zone. Further upstream, starting 40 km from the Scheldt mouth, the detailed qualitative composition was still the same, but a subtle increase in the smectite content was found. Zeelmaekers draws the logical conclusion that the suspended particulate matter (SPM) in the mud plate area and the Western Scheldt area forms one well-mixed continuum in which the marine SPM is transported up the estuary. He further concludes that there are in fact two different types of SPM in the Scheldt estuary: marine-dominated SPM in the saline Western Scheldt and riverine-dominated SPM in the more upstream brackish Middle and fresh Upper Estuaries. This agrees with previous findings that the SPM in the marine part of the Scheldt estuary has a very high marine character (>80%; Van Alphen, Reference Van Alphen1990; Verlaan et al., Reference Verlaan, Donze and Kuik1998; Baeye et al., Reference Baeye, Fettweis, Voulgaris and Van Lancker2011).
Table 22. Clay mineralogy (as ranges in wt%) from mudflats on Belgian Coastal Shelf, Scheldt river and estuary, and marine recent and older deposits from the North Sea and the Netherlands (derived from Zeelmaekers (Reference Zeelmaekers2011) and Adriaens (Reference Adriaens2014)).

5.2.2 North Sea seabed sediments
The clay mineralogy analysis of the surface sediments of the North Sea shows a high heterogeneity in the distribution of the individual clay mineral groups of smectite, illite, kaolinite and chlorite. Using 500 samples, Irion & Zöllmer (Reference Irion and Zöllmer1999) studied the clay mineral composition of the <2 µm fraction of the generally coarse surface sediments of the North Sea and constructed maps of the distribution of the clay minerals illite, chlorite, smectite and kaolinite in these surface sediments (Fig. 10). The smectite content varies between 5 and 53% (Table 6). Almost half of the samples belong to the 20–30% smectite category and are present in the central area, in large parts of the southern North Sea and in the Norwegian Channel as well. Smectite contents of 30–40% are distributed in a narrow zone along the Dutch coast and along the coast of East Anglia, where they co-occur with smectite contents of more than 40%. The illite distribution varies between 34% and 67% (Table 6). The highest fractions are found in the very north and in two areas north and south of the mouth of the river Rhine. Kaolinite ranges from 2% to 23%. Low contents are found in the northwestern part and along the southern and eastern North Sea coast (including the Dutch coast). Along the Dutch coast, the chlorite percentages vary between 10% and 15%.

Fig. 10. Maps of the fractions of different clay minerals in the clay fraction of seabed sediments in the North Sea (redrawn from Irion & Zöllmer, Reference Irion and Zöllmer1999).
It is assumed that the distribution pattern of the different clay mineral associations indicates the origins and pathways of the suspended matter in the North Sea. As in the case of the heavy mineral associations in the surface sediments (Baak, Reference Baak1936; Schüttenhelm & Laban, Reference Schüttenhelm and Laban2005), the heterogeneity is attributed to the various sedimentological processes which have occurred from the Middle Pleistocene to the present day. During cold Pleistocene periods, large ice masses carried illite- and chlorite-rich tillites from the north into the North Sea Basin. This material originated from the Fennoscandian Shield and was deposited in the entire basin during the Elster and Saale glaciations, whereas during the Weichsel only the northern part was covered by the ice sheets (Streif, Reference Streif1990), therefore the fractions of illite and chlorite are higher in the north. On the basis of the literature and their own data, Irion & Zöllmer (Reference Irion and Zöllmer1999) concluded that it seems unlikely that the North Atlantic is an effective source of sediments for the North Sea. The composition of the mud in the Skagerrak and the Norwegian Channel differs greatly from that of mud in the other regions of the northern North Sea (lower chlorite and higher smectite and kaolinite contents). On the west coast of the North Sea (East Anglia), the most striking phenomenon is the relatively high kaolinite content of the sediments. The kaolinite originates mainly from Weichselian tillites formed from Pleistocene sediments and Tertiary soils. On the East Anglia coast there is intensive cliff erosion (McCave, Reference McCave1987), which delivers kaolinite-rich material directly to the sea. The distribution of kaolinite-rich sediments corresponds with the calculations and remote sensing data of McManus & Prandle (Reference McManus and Prandle1997). The high smectite values along the Belgian and Dutch coasts are explained as the erosion products of the smectite-rich Cretaceous formations of the Dover Strait. About 50% of these Dover Strait sediments are transported by longshore drift along the Belgian–Dutch coast, some reaching the German Lower Saxony coast (Salomons, Reference Salomons1975; Salomons et al., Reference Salomons, Hofman, Boelens and Mook1975; Van Alphen, Reference Van Alphen1990; McManus & Prandle, Reference McManus and Prandle1997; Brockamp & Clauer, Reference Brockamp and Clauer2012).
Irion and Zöllmer (Reference Irion and Zöllmer1999) attribute the large amounts of smectites in the German Bight to the Elsterian glaciation. This material was later mixed with illite- and chlorite-rich sediments from the north but nonetheless remained rich in smectite. Using mass-balance calculations, Hoselmann & Streif (Reference Hoselmann, Streif and Streif2004) calculated that 90% of the sediment volume along the Lower Saxony coast originates from submarine erosion of the seafloor whereas only 10% originates from the Ems, Weser and Elbe rivers. Müller & Forstner (Reference Müller and Forstner1975) and Irion et al. (Reference Irion, Wunderlich and Schwedhelm1987) even assumed transport of fine-grained uncontaminated sediments from the North Sea as far as Hamburg in the Elbe estuary and a decreasing mixing with the fluvial suspension load to explain the upstream increase in heavy metal contents of the river sediments. By contrast, on the basis of the K/Rb and Mg contents of the illite, Zuther et al. (Reference Zuther, Brockamp and Clauer2000), Pache et al. (Reference Pache, Brockamp and Clauer2008) and Brockamp & Clauer (Reference Brockamp and Clauer2012) determined that at least 80% of the sediments off the Weser and Elbe estuaries and nearby coastal areas are of fluvial origin and the rest of marine origin. According to these authors, there seems to have been no transport of large proportions of fine-grained sediment from the North Sea into the Weser and Elbe estuaries. Thus the origin of clay minerals in this coastal region remains unclear. However, it is generally agreed that the rate of accumulation in an estuary will depend on the balance of sediment retention to sediment export. This balance will depend on the strength of the sediment transport during ebb tide, which will become less effective with long estuarine residence times, as is the case for the Weser (45 days), Elbe (84 days) and Scheldt (90 days) estuaries (Uncles et al., Reference Uncles, Stephens and Smith2002). This means that it is unlikely that these rivers transport large quantities of fluvial clays into the North Sea.
5.2.3 Palaeogene and Neogene formations
Several studies have paid attention to the clay mineralogy of Palaeogene and Neogene deposits, in particular to the Rupel Clay (also referred to as the Boom Clay in the Netherlands and Belgium). Table 23 summarises the analytical results. The results of Zeelmaekers (Reference Zeelmaekers2011) and Koenen & Griffioen (Reference Koenen and Griffioen2013) were obtained using a new quantitative approach developed by Zeelmaekers (Reference Zeelmaekers2011). The data of Kuhlmann et al. (Reference Kuhlmann, De Boer, Pedersen and Wong2004) on Pliocene and also Early Pleistocene sediments originates from cores taken in the northern part of the Dutch continental shelf and the rest originates from onshore drillings or quarries (note that the Gelasian stage that was studied is nowadays classified under Pleistocene instead of Pliocene). Kuhlmann's data indicates very variable clay mineral assemblages along the depth profiles: the fractions vary by 50% for three clay minerals, which indicates large heterogeneity within this 600 m long sequence. The results for the Rupel or Boom Clay are reasonably consistent, except for the high kaolinite fraction and the low interstratified illite/smectite and smectite fractions found by Heederik et al. (Reference Heederik1989). The Ypresian clay has larger fractions of smectite and interstratified illite/smectite than the rest (Zeelmaekers, Reference Zeelmaekers2011).
Table 23. Clay mineralogy (in wt%) of Palaeogene and Neogene marine sediments studied in the Netherlands or Belgium. All results are based on the <2 μm fraction of the samples except Decleer et al. (Reference Decleer, Viaene and Vandenberghe1983), which are based on whole sediment.

The shifts in clay mineral assemblage at the geological time scale were primarily explained in terms of provenance control by both Kuhlmann et al. (Reference Kuhlmann, De Boer, Pedersen and Wong2004) and Zeelmaekers (Reference Zeelmaekers2011). Climate factors seem to affect the contribution of individual hinterlands, but the climate dependency of weathering of clay minerals is believed to be of minor importance. According to Zeelmaekers (Reference Zeelmaekers2011), the high smectite fraction in the Ypresian clay can be attributed to a contribution of basic pyroclastic material related to the opening of the North Atlantic and connectivity with the North Sea (cf. Nielsen et al., Reference Nielsen, Rasmussen and Thyberg2015). A remarkable difference between these smectites and the other clays in the samples studied is that the smectites are authigenic whereas the other clay minerals usually have a detrital origin. The shifts in the clay mineral assemblage of the Late Pliocene record have been attributed to shifts in the supply of predominantly illite and chlorite from Scandinavian sources during cold periods versus predominantly smectite from southern sources during warmer conditions. Shifts in the clay mineral assemblage can also be recognised in the Rupel or Boom Clay. Zeelmaekers (Reference Zeelmaekers2011) noted a higher kaolinite fraction for the more clayey intervals than the more silty intervals, following a Milankovitch cyclic pattern; Koenen & Griffioen (Reference Koenen and Griffioen2013) noted more kaolinite/smectite mixed layers and smectite in the samples that had a higher clay content with less interstratified illite/smectite and illite.
5.3 Feldspars, micas and silica
5.3.1 Feldspars and micas
The occurrence of feldspars and micas in marine sediments has received little attention, as is also the case for fluvial sediments. Druif (Reference Druif1927) observed all three feldspars, orthoclase, microcline and plagioclase, microscopically in two Holocene marine sand samples and found orthoclase to be the most abundant. Muscovite and biotite were found together in one sample but not in the other one. For three Tertiary sand samples in Limburg, he observed orthoclase and muscovite, no biotite and plagioclase and few or very few microcline in the two Oligocene samples. Using XRD, Bouezmarni & Wollast (Reference Bouezmarni and Wollast2005) identified albite and microcline in bottom sediments of the Scheldt: the amounts were higher in the 8–63 μm fraction than in the <8 μm fraction. Also based on XRD analysis, Koenen & Griffioen (Reference Koenen and Griffioen2013) reported 0.3–5.6% plagioclase and 2.2–11% K feldspar for the Rupel Clay in a series of samples from across the Netherlands. Decleer et al. (Reference Decleer, Viaene and Vandenberghe1983) found higher contents of 9.8–15.7% for the unit in Belgium. Both studies indicate substantial presence of the minerals and more prominent presence of K feldspar over Na feldspar or Ca feldspar. The most extensive investigation on feldspars and micas in the marine environment we are aware of was performed by Crommelin (Reference Crommelin1943). He made optical mineral counts of the light minerals in suspended matter and sediments of the Wadden Sea and some other water systems, often characterising individual grain size classes. Table 24 presents some of the results. The data show the dominance of orthoclase, albite and oligoclase, and minor roles of microcline and Ca-rich feldspar. The overall results indicate that biotite, chlorite and/or muscovite are omnipresent in the 10–50 μm fraction and much less in the >50 μm fraction in the marine environment. A comparison between the Wadden Sea, North Sea and river samples indicates that the first two are very similar and the river samples are richer in micas and chlorite. Finally, Huisman & Kiden (Reference Huisman and Kiden1998) used the Na2O content of sediment as an indicator of Na feldspar content. They reported Na2O-rich marine sediments of the Oosterhout Formation versus Na2O-poor marine sediments of the Oosterhout and Breda Formations. This difference was attributed to a different weathering intensity and shifts in the provenance. Similar differences were observed for the fluvial sediments from the same geological period (see section 4.3).
Table 24. Mineral counts of feldspars and micas (out of 100 grains of light minerals) in sediments and/or suspended matter in the Wadden Sea, the North Sea and the Rhine river (calcite and aragonite were removed prior to counting; derived from Crommelin (Reference Crommelin1943)).

5.3.2 Biogenic silica
Quartz is abundant in Dutch sediments. Several kinds of biogenic silica may also be present in sediments: diatom valves, radiolarian, sponge spicules phytoliths and chrysophyceae. Diatoms are frequently studied within the context of chronostratigraphy and palaeoenvironmental reconstructions, but absolute contents – the finding most interesting for our review – are not often determined. They are a constituent of fresh and marine phytoplankton and consist of amorphous hydrated particulate silica. Table 25 presents an overview of the amounts of biogenic silica in suspended matter of the Scheldt system and of diatoms in marine or other sediments. The old data of Crommelin (Reference Crommelin1945) agree fairly well with more recent data of Cremer et al. (Reference Cremer, Bunnik, Kirilova, Lammens and Lotter2009, Reference Cremer, Bunnik, Donders, Hoek, Koolen-Eekhout, Koolmees and Lavooi2010) and Sabbe (Reference Sabbe1993). The typical diatom concentration ranges from 1 to several hundreds of valves/gsed. It appears that lacustrine sediments contain the highest concentrations: this fits in with the general observation that lakes are efficient silica traps (Street-Perrott & Barker, Reference Street-Parrott and Barker2008). The actual concentration depends on the local production versus the external sediment load and possibly compaction. An amount <10 valves/gsed has been found for both peat and Potklei, but this finding cannot be generalised, for reasons discussed in section 6. The fraction of biogenic silica in the suspended matter of the Scheldt system varies around several per cent. Both Sabbe (Reference Sabbe1993) and Carbonnel et al. (Reference Carbonnel, Vanderborght, Lionard and Chou2013) found that the concentration of free living diatoms is small by comparison with that associated with suspended matter or sandy sediment grains (and which is largely dead material). On the intertidal flat Sabbe studied, the living diatoms showed strong temporal variations and correlations with sky radiance and percentage organic matter, whereas the dead diatom mass appeared to be stable (Sabbe, Reference Sabbe1993). Similarly, the temporal and longitudinal variability of biogenic silica in the Scheldt system was highly related to the variability of the suspended matter, with the lowest fraction occurring in the zone with intermediate salinity (Carbonnel et al., Reference Carbonnel, Vanderborght, Lionard and Chou2013).
Table 25. Amounts of diatoms in different kinds of Dutch geological sediments.

The solubility of biogenic silica is about 50 mg Si/L, which is 17 times higher than that of quartz (Cornelis et al., Reference Cornelis, Delvaux, Georg, Lucas, Ranger and Opfergelt2011). Dissolution of biogenic silica in buried sediments may thus be an important source of dissolved silica in groundwater (Miretzky et al., Reference Mitetzky, Conzonno and Fernandez Cirelli2001). The weight of diatom valves varies around 5 × 10–11 g SiO2/valve (Sané et al., Reference Sané, Isla, Angeles Barcena and DeMaster2013). A concentration of 10 million valves/gsed thus corresponds to 500 ppm Si. If the recharge is 350 mm/y and no other silica source is present, it would thus take 23 years for a layer of sediment 1 m thick to be totally depleted of biogenic silica. Leaching of biogenic silica from sediments could therefore be a relevant process at the geological time scale in infiltration areas.
5.4 Ca carbonates, phosphates and gypsum
5.4.1 Contents
Calcium carbonates are generally observed in marine deposits and these deposits may also contain shells or shell fragments. In young marine sediments from various coastal areas, Verhoeven (Reference Verhoeven1962) noted an increase in carbonate content from a few per cent to 10–22% with increasing clay content until 15–30% clay and a constant content above these levels. The highest contents were found near the Western Scheldt and the lowest at the Zuiderzee, which agrees with the general pattern of CaCO3 decreasing northwards from the French coast to the Danish coast. Table 26 presents percentile data on carbonate contents of marine geological formations, mostly obtained by CS elemental or thermogravimetric analysis. Clay and loam have somewhat higher carbonate contents than sand. An important exception is the Oosterhout Formation, which has high carbonate contents in the sand fraction. This is due to the presence of a Crag facies with over 15% Ca carbonate, which is classified as sand (Keijzer & Griffioen, Reference Keijzer and Griffioen2012). Crag deposits are sand containing abundant shells and bryozoans; these sediments form the lower part of the Oosterhout Formation in southern and northeastern Netherlands (www.dinoloket.nl). The numbers for the Boom Clay (Decleer et al., Reference Decleer, Viaene and Vandenberghe1983) suggest that the carbonate concretions present were not included. Koenen & Griffioen (Reference Koenen and Griffioen2013) also observed that the calcite content in the Rupel Clay is mostly about equal to (<10% difference) or greater than the aragonite content: the maximum calcite content is 25.9% and the maximum aragonite content is 5.1%. Klein & Griffioen (Reference Klein and Griffioen2012) showed that in the northern Netherlands the carbonate content in the Late Pleistocene Eem Formation is significantly lower than in the Holocene Formation for both clay and sand. These two findings indicate that at the regional scale the marine formations differ substantially in terms of their carbonate characteristics.
Table 26. Regional characteristics (as percentiles) of the pyrite, reactive Fe, organic matter and Ca carbonate contents in several Dutch marine geological formations. Geotop refers to the first tens of metres below surface and 5a2 and 5b2 are two of these areas (see Van Gaans et al. (Reference Van Gaans, Griffioen, Mol and Klaver2011) and Griffioen et al. (Reference Griffioen, Vermooten and Janssen2013)). Note that 82.5 percentile is given in regular or 90 percentile in italic script.

~ As organic C instead of organic matter
Zwolsman (Reference Zwolsman and Van Eck1999) presents the carbonate contents of suspended matter in the Western Scheldt: about 28% for the marine end member, with larger amounts in the winter half year than in the summer half year. A similar gradient was found by Zwolsman (Reference Zwolsman1994) for suspended matter: the CaCO3 fraction is about 20% for salinity 15 PSU and higher and about 10% for 0.5 PSU (PSU is practical salinity unit and is a measure of the electrical conductivity of seawater; ocean water has a PSU of approximately 35, being near-equivalent to 35 g salt/kg).
The highest CaCO3 content is found in the silt fraction of young sediments: more specifically, in the 2–8 μm and 8–16 μm fractions (Verhoeven, Reference Verhoeven1962; Salomons, Reference Salomons1975) or the 16–43 μm fraction (Zuur, Reference Zuur1936). The clay fraction proves to be relatively poor, having CaCO3 contents that are four to six times lower. The CaCO3 content of the 75–1400 μm and >1400 μm fractions varies somewhat depending on the presence of shell fragments. The material suspended in the Dutch Wadden area and the newly deposited sediments showed nearly the same distribution of CaCO3 over the various grain size fractions. We assume that this is probably true for samples that contain only detrital carbonate and no or few authigenic shell fragments, since the shell fragments are usually larger than 200 μm (Eisma, Reference Eisma1968). Conclusions on carbonate gradients for young sediments within the southwestern estuaries are conflicting. Verhoeven (Reference Verhoeven1962) and Salomons (Reference Salomons1975) conclude that the CaCO3 content of estuarine deposits of the Western Scheldt decreases with decreasing salinity, where the relation between content and grain size class remains about the same. Beeftink et al. (Reference Beeftink, Daane, Van Liere and Nieuwenhuize1977) do not observe a significant correlation between carbonate content in tidal marshes and distance from the North Sea. They attribute this to differences in diagenetic decalcification under different local environmental conditions that may obscure the regional depositional gradient.
Note that prior to routine grain size analysis, carbonate is often removed together with organic matter because authigenic carbonate may cement individual grains into aggregates. This removal has two implications: (1) for many kinds of calcareous sediment there is a systematic difference in grain size distribution between original sediment and analysed sediment, and (2) in a considerable fraction of carbonate-rich sediment the true grain size distribution is unclear. Geologists are traditionally interested in the grain size distribution of the ensemble of individual grains. However, for hydrogeologists and geochemists the actual grain size distribution may be more relevant, for example in order to deal with permeability and carbonates.
It is worth pointing out that Druif (Reference Druif1927) microscopicaly observed apatite in ‘normal amounts’ in two marine sand samples. Remarkable is the presence of U-rich phosphorite nodules in sands of the Breda Formation in Zeeland (Harseveldt, Reference Harseveldt1973). Based on 12 samples, the content of Ca phosphate varies between 25 and 69 wt% expressed on the basis of Ca3(PO4)2. The layers containing the nodules appear to be discontinuous. The nodules are composed of light green glauconite globules, quartz grains and some feldspar grains embedded in a cryptocrystalline matrix of apatite.
5.4.2 Provenance of carbonates
Calcium carbonate in marine deposits may originate from fluviatile carbonates, carbonates derived from eroded carbonate formations along the coast (as in the Dover Strait), recent biogenic carbonates (such as shells) and also authigenic carbonate (Salomons, Reference Salomons1975; Kooistra, Reference Kooistra1978; Fig. 11). Salomons (Reference Salomons1975) characterised recent marine and estuarine carbonates according to their mineralogical and isotopic composition, using sediment samples with 50% of the grains less than <20 μm. The recent biogenic carbonates are high-Mg calcite and aragonite, both having a high 14C content. Salomons could not detect aragonite and he found that the average 14C content of recent sediments in the Wadden Sea area or southern part of the North Sea varied around 24–36 PMC. This implies that recent biogenic carbonate is not an important constituent of these recent marine sediments as its radiocarbon content is close to 100 PMC. The δ13C value was around 0‰ whereas that for the recent Rhine-Meuse sediments was –3.5‰ and for the Ems sediments it was –0.5‰ (Table 11). This is further evidence that the recent marine sediments mainly originate from eroded carbonate formations along the coast. Salomons (Reference Salomons1975) estimated the contribution from the rivers Rhine and Meuse to the sediments in the Wadden Sea is no more than 20%. Verhoeven (Reference Verhoeven1962) mentions a microscopy study of Favejee on the biogenic CaCO3 particles in various grain size classes. The bulk of the particles could not be determined, but shell fragments, Foraminifera, Ostracoda and species of Echinodermata were found, and the fine fractions below 16 μm in particular contained some rhombohedral crystals and fine, needle-shaped particles. By contrast, Bouezmarni & Wollast (Reference Bouezmarni and Wollast2005) found calcite and aragonite in comparable amounts in superficial sands of the Scheldt estuary and only calcite in the muddy sediments. Micromorphological investigation indicated that the latter calcite occurred as rhombohedral crystals, which were authigenically formed particularly in relation to sulphate reduction within anoxic zones of the estuary.

Fig. 11. Major sources and fluxes of Ca-carbonates in the Rhine-Meuse-Scheldt delta area and related southern North Sea.
The carbonates in the Rhine-Meuse estuary before closure by the Delta works and the Ems estuary had a high marine fraction. According to Salomons (Reference Salomons1975), the proportion of carbonate derived from a marine environment was more than 40% for the Hollands Diep, about 70% for the Haringvliet and up to 90% for the Ems estuary. Similarly, the results of Verlaan et al. (Reference Verlaan, Donze and Kuik1998) show that Ca as carbonate in suspended matter of the Scheldt estuary is predominantly of marine origin and its influence extends throughout the entire brackish-water zone. δ13C analysis of carbonate in samples from the marine Oosterhout and Breda Formations also yielded values close to zero, which points to a marine origin for these much older Neogene deposits too (Hartog et al., Reference Hartog, Griffioen and Van Bergen2005).
5.4.3 Shells
Shells are authigenically grown Ca carbonates which are commonly found in marine sediments. The topic of shell production has received considerable interest in the context of sustainable harvesting of shellfish and of shells from the Wadden Sea (e.g. Beukema & Cadée, Reference Beukema and Cadée1999; De Vries, Reference De Vries2000). The dominant shells in the Wadden Sea originate from cockles (Cerastoderma edule), which make up 75–90% of the total volume of shell produced. The average annual amount of Ca carbonate produced by adult cockles is 84 g/m2, amounting to a total of 126 million kg/y in the Dutch Wadden Sea (Beukema & Cadée, Reference Beukema and Cadée1999). This production rate is equivalent to 189,000 m3/y, whereas the sedimentation rate at the Dutch Wadden Sea is 9.4 million m3/y (Elias et al., Reference Elias, Van der Spek, Wang and De Ronde2012). The ratio between these two rates is 0.02, i.e. the authigenically grown Ca carbonate production is substantial when compared to the Ca carbonate content of a few per cent usually found in marine or estuarine sediments (Table 26).
The destiny of these shells varies: nowadays about 5% are removed by fishery, an estimated 5% become more or less permanently buried, about 17% are fragmented by birds (eider ducks in particular) producing shell fragments of mostly 2–8 mm and the large remaining proportion is transported to tidal streams, where they form dense accumulations at the bottom (Fig. 12). Beukema & Cadée (Reference Beukema and Cadée1999) considered physical fragmentation and chemical dissolution to be negligible in the sheltered Wadden Sea environment. Cockle shells are transported only during severe storms, when sufficient energy is present to lift and transport them (De Vries, Reference De Vries2000). The horizontal distances travelled are therefore small. A more important transport mechanism is erosion of intertidal flats by shifting gully channels: the shells become concentrated at the bottom of the gully and the sand is transported away. This results in the formation of shell beds, which may become buried by more permanent movement of gullies at the geological time scale. Geologically, the shells, their fragments and the associated sand may become preserved as channel lag deposits.

Fig. 12. Sediment cores from drilling B19C0473 at Bakkum (North-Holland) showing in the central core tidal stream deposits with shell fragments on top of a peat layer that features the bioturbation burrows of a mussel.
5.4.4 Authigenic carbonate and decalcification
Both neo-formation of carbonate and decalcification were observed by Kooistra (Reference Kooistra1978) in recent estuarine sediments. Neo-formation is common in the intertidal flats and decalcification in the medium-high and high supratidal flats. Two morphologies were found for the neoformation: (1) single rhombohedral crystals in the soil matrix and (2) accumulations of fine particles associated with voids. Local evaporation caused the carbonate to precipitate from oversaturated solution. Decalcification is strongly associated with pyrite oxidation, as will be discussed later.
The Boom Clay contains septarian carbonate concretions in several stratigraphical levels (De Craen et al., Reference De Craen, Swennen and Keppens1999a,b; Fig. 13), which are always associated with thin bioclast-rich layers. The authigenic carbonate that forms the concretions is dominantly calcite. Siderite is also prominently present in two stratigraphically distinguished horizons. The concretions consist of microcrystals of 1–5 μm with associated amounts of pyrite. The oxygen isotopic composition (Table 11) suggests precipitation under marine conditions in association with degradation of organic matter. The organic geochemistry indicates a strong terrigenous input of organic matter and the presence of bacterial mass. The observation that delicate sedimentologial features remained preserved in the concretions indicates that concretions must have grown early in diagenesis. De Craen et al. (Reference De Craen, Swennen and Keppens1999a) concluded that the concretions were formed due to microbially mediated sulphate reduction with related precipitation of pyrite and calcite, and – to a lesser extent – iron and manganese oxide reduction.

Fig. 13. Example of a septarian carbonate concretion in the Boom Clay as observed in a quarry near Leuven (Belgium).
A special phenomenon is the presence of so-called pseudo-gaylussite (also known under other names such as glendonite), which is typically found in a depth range of one to several metres in young Holocene marine clay deposits of the provinces North Holland, Friesland and Groningen (e.g. Van Baren, Reference Van Baren1926; Oenema, Reference Oenema1990c). It mostly appears as elongated monoclinically shaped porous, brittle crystals 1–2 cm long and ochre-yellow in colour. They are mainly composed of calcite, which must be pseudomorphologically substituted because calcite has a trigonal-rhombohedral crystal structure. Shearman & Smith (Reference Shearman and Smith1990) proposed that the parent mineral is not gaylussite but ikaite. The composition of gaylussite is Na2Ca(CO3)2•5H2O and it is typically found associated with evaporites in closed arid basins, as in the Kenyan Rift Valley. Ikaite is composed of CaCO3•6H2O and found in cold, estuarine or marine environments such as Greenland. The latter environment is more similar to Dutch conditions, where so-called pseudo-gaylussite and the brittle, porous structure can be explained by loss of crystal water during recrystallisation from ikaite to calcite (Selleck et al., Reference Selleck, Carr and Jones2007). Ikaite precipitates preferentially over other CaCO3 polymorphs in the presence of elevated alkalinity and dissolved phosphate. Such conditions are found in the coastal provinces of the Netherlands, where they are associated with freshening of marine sediments (Griffioen et al., Reference Griffioen, Vermooten and Janssen2013).
5.4.5 The occurrence of dolomite
Several researchers have studied the occurrence of dolomite in addition to calcite, using XRD, microscopy after pretreatment with HCl to remove calcite or selective extraction in acid solution. XRD analysis revealed that the carbonates in the sediments of Hollands Diep, Haringvliet, Ems estuary and the Wadden Sea consisted of low-Mg calcite (Mg content <1%) and dolomite, with the dolomite fraction accounting for about 7% of total carbonate (Salomons, Reference Salomons1975). The percentage of CO2 bound to Mg is generally about 10% but may vary up to 22% (Verhoeven, Reference Verhoeven1962) or even 30% (Bruin, Reference Bruin1938) for soil samples from various regions. In grain size classes >10 μm, Crommelin (Reference Crommelin1943) found a widespread occurrence of dolomite in both suspended material and young sediments of the North Sea and Dutch Wadden Sea. The dolomite content can be high locally: up to 10% of total carbonate or even more in the 10–25 and 25–50 μm classes of samples decalcified prior to microscopical investigation. Dolomite thus mainly occurs in the silt fraction. Morphologically, it occurs as small aggregates or individual rhombohedral crystals. Since little or no dolomite was found in similar grain size classes of suspended material sampled from the rivers Ems and Rhine, Crommelin proposed dolomite as a proxy mineral for marine sediment. The <2 μm fraction of samples from marine or estuarine soils contains 1–6% carbonates (Van der Marel, Reference Van der Marel1950). Substantial amounts of dolomite (up to 15% of total carbonate) were found in tidal flats on the northern coast and negligible amounts (3% or less of total carbonate) in soils in the southwestern delta area. The difference was attributed to a difference in the origin of the parent material: Scandinavian versus French and Belgian. It is worth noting that the <2 μm fraction and the 2–16 μm fraction are strongly correlated in marine sediments (Kooistra, Reference Kooistra1978; Zuur, Reference Zuur1954) because the finer particles are mostly transported in mud flakes, in which these fractions are present in constant ratios. The dolomite content, however, is distinctly different: high in the 2–16 μm fraction and low in the <2 μm fraction. The relationship of calcite and dolomite with grain size thus differs both between these two minerals and also geographically.
5.4.6 Carbonate in beach sand
The two kinds of beach sand along the coast of North and South Holland are also distinguishable by their carbonate content. The CaCO3 content of beach sands north of Bergen is usually less than 0.25% whereas south of Castricum the content is usually 2% or more (Eisma, Reference Eisma1968). The highest contents (up to 12%) are found between Wijk aan Zee and Noordwijk. The content of the separate sand fractions is lowest in the 250–315 μm fraction and increases towards the finer and the coarser fractions. The carbonate content is lower again on the beaches of the islands of South Holland and Zeeland: it may be as low as <1%. Hot HCl extractable Mg, which can largely be attributed to carbonates, is around 0.1% MgCO3 north of Bergen and around 0.4% MgCO3 south of Bakkum (Eisma, Reference Eisma1968). There is also a north–south difference in Ca to Mg ratio: it varies around 3–4 for the northern type of beach sands and around 8 for the southern type. Both the low carbonate content and the low Ca to Mg ratio for the northern sands are due to (1) prolonged leaching during Middle Pleistocene to Middle Holocene under terrestrial conditions with preferential dissolution of Ca from carbonates rather than Mg and (2) continuing erosion of the coast north of Bergen with little recent deposition of shell fragments on the beach. Another factor controlling the Mg content and its ratio to Ca content is the presence of Echinoid fragments, which have a higher Mg content than mollusc shell fragments. It is interesting that the shell fragments present have a lower size limit of about 200 μm while detrital calcite is mainly present in the finer fractions (Eisma, Reference Eisma1968). The shell fragments are formed by biogenic fragmentation of the fragile mollusc shells; physical fragmentation by currents and waves seems to be of minor importance in this environment. The shell fragments do not remain where they were produced but instead accumulate on stable or advancing beach stretches, i.e. south of Bergen. The distribution of whole dead shells and fragments >1000 μm in marine sediments is similar to the distribution of living molluscs, except in the case of a few local secondary accumulations, where finer clastic material has been removed.
5.4.7 Decalcification
Decalcification is observed at shallow depth in supratidal sediments, probably caused by cyclic oxidation of pyrite (Kooistra, Reference Kooistra1978). This early diagenetic process may give rise to carbonate deficits in middle-high and high salt marshes. Since no gypsum was observed by Kooistra at these sites (which is in contrast to the findings on reclaimed marine soils), this suggests that good drainage or sufficient washing with sea water has prevented gypsum accumulation. Zwolsman et al. (Reference Zwolsman, Berger and Van Eck1993) described two shallow carbonate profiles in brackish supratidal flats: immediately below the surface the carbonate content dropped by 1% in one profile and by 5% in the other. At 0.1–0.3 m depth it increased by 3% in the first profile and by 6% in the second profile. The drop happens within the oxic zone and is attributed to a transient drop in pH resulting from decomposition of organic matter, denitrification and oxidation of iron sulphides. The increase at the greater depth, which was in the reduced zone for one profile studied, is attributed to authigenic carbonate precipitation following an increase in alkalinity on sulphate reduction. The increase in the second profile remained unexplained. Comparably, Vranken et al. (Reference Vranken, Oenema and Mulder1990) report decalcification rates of 2–8 moles of CaCO3/(m2.y) for supratidal sediments of the Eastern Scheldt due to predominantly pyrite oxidation. This is equivalent to 0.2–0.8% CaCO3/y for a 20 cm thick decalcification zone and dry bulk density of 500 kg/m3 as reported. Substantial decalcification and probably also calcification may thus happen in estuarine sediments in an early diagenetic stage prior to geological burial, from which it can be concluded that the Ca carbonate content of these geological sediments is not only controlled by deposition.
Van der Sluijs (Reference Van der Sluijs1970) put the decalcification of supratidal flats in a geomorphological perspective. He observed that little decalcification occurred below unvegetated areas whereas decalcification below vegetated parts was high – sometimes even higher than in reclaimed marine clay soils: loss of 12% CaCO3 per century over a depth of 40 cm or 6% per century over a depth of 55 cm. The thickness of the zone deficient in Ca carbonate was observed to be usually less than 40 cm, but thicknesses up to 80 cm were also observed. He distinguished the levees along the creeks and the pools further inland. In the levees, decalcification is more favourable because of more sandy sediment, greater permeability and less carbonate input during incidental flooding and subsequent settling of marine, calcareous mud. He also explains why thin marine deposits that wedge out against the Pleistocene landscape are generally carbonate-free: such areas are vegetated supratidal flats where conditions for decalcification are favourable and input of marine Ca carbonate is limited due to incidental flooding.
Ritsema & Groeneberg (Reference Ritsema and Groenenberg1993) described a soil profile of a marine soil in a polder in South Holland, where at 82 cm depth both calcite and dolomite are present, at 25 cm depth only dolomite is present and at 2.5 cm depth both carbonates are absent. This points to the selective dissolution of initially calcite and subsequently dolomite, which is in line with the dissolution kinetics of these two carbonate minerals (Morse & Arvidson, Reference Morse and Arvidson2002). The rate of decalcification of reclaimed marine soils will vary depending amongst others on the intensity of pyrite oxidation and its pH buffering by carbonate (see later). A representative value is provided by Edelman & De Smet (Reference Edelman and De Smet1951), who suggested 1% of CaCO3 per 65–90 years for a layer 40 cm thick.
Subregional patterns in carbonate content have been observed for marine clay soils and attributed to prolonged decalcification after cultivation. For example, Spijker (Reference Spijker2005) separates topsoils in Zeeland into calcareous heavy clays and non-calcareous heavy clays based on XRF analyses of Al and Ca as proxies for clay content and carbonate content, respectively. Within Zeeland province, the calcareous heavy clays are found in southern Noord-Beveland and western Zeeuws Vlaanderen, whereas the non-calcareous heavy clays occur in Walcheren and Schouwen-Duiveland. Care must be taken to attribute differences in carbonate content between polders to the duration of cultivation after reclamation, as the parent material may also differ in carbonate content (Edelman & De Smet, Reference Edelman and De Smet1951; Van der Sluijs, Reference Van der Sluijs1970).
Gypsum may be found at shallow depth in calcareous, marine soils of northern and western polders. It is authigenically formed by extensive oxidation of pyrite and pH buffering by Ca carbonate dissolution. Miedema et al. (Reference Miedema, Jongmans and Slager1973) observed gypsum at a depth of 1 m, which is the deepest depth of the seasonally fluctuating groundwater table. SEM investigation showed that it occurred as secondary crystals along large channels or as crystal tubes filling channels, through which oxygen enters the soils. The amount of gypsum probably depends on the initial Ca carbonate content versus pyrite content. Prolonged cultivation of soils containing secondary gypsum may dissolve all gypsum formed.
5.5 Reactive Fe minerals: sulphides, oxyhydroxides, carbonates and glauconite
5.5.1 Fe oxyhydroxides
As was the case for fluvial sediments, mineralogical studies on Fe oxides in marine sediments are rare. Most attention has been paid to the occurrence of oxides in relation to sulphide oxidation, which is discussed in the next section. Nelson & Niggli (Reference Nelson and Niggli1950) isolated opaque grains and performed XRD analysis on two samples of beach sand and one Early Holocene marine sand from further inland. In the beach samples, ilmenite and rutile were dominant in about similar proportions and together comprised 80% of the heavy mineral fraction. Magnetite and little ilmenite were present in the magnetic subfraction. In the inland sample, no Fe oxides were observed as opaque minerals but pyrite was. This illustrates that diagenesis of reactive Fe minerals also might affect the opaque mineral fraction of sediments.
As mentioned earlier, two types of beach sand are distinguished in the Netherlands based on mineralogical composition. Dithionite-extractable Fe is around 0.03% Fe2O3 north of Bergen and around 0.10% south of Bakkum (Eisma, Reference Eisma1968), which is relatively low (cf. Table 13). This Fe occurs as Fe oxide coating also because the Fe in heavy minerals is negligibly soluble in dithionite. XRD analysis has shown that goethite is present but amorphous material is more abundant. Eisma (Reference Eisma1968) related the difference to release from sediment particles in the Rhine-Meuse estuary and subsequent flocculation of Fe hydroxides with longshore transport and sedimentation, predominantly as far as IJmuiden. This release might be associated with degradation of fluvial organic matter under marine conditions (Eisma, Reference Eisma1986). Van Straaten (Reference Van Straaten1954) mentions that gravel-sized concretions of iron hydroxides occur incidentally at the foot of marsh cliffs, where they became concentrated after erosion of the salt marshes. They were mainly formed in these marshes at faunal channels (see section 5.5.3).
Dehairs et al. (Reference Dehairs, Baeyens and Van Gansbeke1989) and Turner et al. (Reference Turner, Millward and Morris1991) addressed the variability of Fe oxides in suspended matter of the Scheldt estuary and North Sea, respectively. Turner et al. (Reference Turner, Millward and Morris1991) used selective oxide extraction and found that extractable Fe varied around 3 mg/g and extractable Mn around 0.7 mg/g (to convert to wt% of Fe2O3, multiply by 0.1436). In the Scheldt samples, Fe had a maximum of about 4 mg/g at 2–5‰ salinity and Mn has a maximum of about 1 mg/g at 15–25‰ salinity. These maxima were explained by oxidation of dissolved Fe(II) and Mn(II) downstream of the anoxic zone in the Scheldt estuary and associated settling, where Fe(II) oxidises more rapidly than Mn(II). Dehairs et al. (Reference Dehairs, Baeyens and Van Gansbeke1989) used non-selective acid leaching and interpreted the variability in Fe/Al and Mn/Al ratios in terms of variability in the contents of Fe and Mn oxides. A seasonal variability was observed, with the lowest ratios occurring during April–May and the highest during October–February. Sediments from the top layer generally exhibited the inverse temporal trend. This fluctuation was attributed to phytoplankton bloom in spring with oxic top sediment during this season, and to mineralisation of fresh settled organic matter with anoxic conditions of the top layer, reductive dissolution of Fe and Mn oxides, related release to the seawater above and subsequent re-oxidation in the water during the rest of the year.
Extractable Fe oxides in the bottom sediments of the southern North Sea and Skagerrak were studied by Slomp et al. (Reference Slomp, Malscaert, Lohse and Van Raaphorst1997). They found the highest contents of around 11,000 mg Fe/kg at the Skagerrak, where there is large input of terrigenous material. The content varied around 3000 mg/kg in the German Bight (where input is also large) and in the zones with sand where erosion is dominant, and it was between 600 and 3000 mg/kg in the zones with fine sands and temporary deposition of organic matter. Slomp et al. (Reference Slomp, Malscaert, Lohse and Van Raaphorst1997) noted that most North Sea sediments are relatively poor in Fe oxides, but comparison with the data in Table 13 indicates that the contents are the same order of magnitude as those for Dutch fluvial sediments.
5.5.2 Fe sulphides
Several researchers have observed that suspended matter and also fresh mud in both the Scheldt area and the Wadden Sea contain up to 1% pyrite (Harmsen, Reference Harmsen1954; Kooistra, Reference Kooistra1978; Verlaan et al., Reference Verlaan, Donze and Kuik1998; Zwolsman & Van Eck, Reference Zwolsman and Van Eck1999). This is attributed to resuspension of recent reduced sediments, erosion of non-recent sediments and dredging activities. Both Verlaan et al. (Reference Verlaan, Donze and Kuik1998) and Zwolsman & Van Eck (Reference Zwolsman and Van Eck1999) noted that the maximum pyrite content is found in the upper, brackish part of the Scheldt estuary (between Rupelmonde and the Dutch–Belgian border), where the salinity is about 5‰. Data from Oenema (Reference Oenema1990a) indicate that the pyrite content in anoxic recent marine sediments can be up to 2–3%, and attribute this to in situ sulphidation. The contents are rather invariant with depth down to 80 cm deep.
Harmsen (Reference Harmsen1954) analysed topsoil samples from polders reclaimed from the sea to ascertain their sulphur contents. He found that some old and well-aerated soils contained significant percentages of pyrite while in others pyrite was absent. No reason for this difference could be observed. He postulated that the difference in reactivity was the result of a retarding process, possibly the formation of insoluble Fe(III) phosphate (cf. Elsetinow et al., Reference Elsetinow, Schoonen and Strongin2001).
Buried marine sediments contain pyrite, as evidenced by their S contents, data from micromorphological investigations and the reactivity of the sediments in aerobic incubations. Table 26 presents statistical data on the pyrite and reactive Fe contents obtained from total elemental analysis. The Dutch samples were classified into lithological class, geological formation, geographic region and sometimes also sedimentary facies. The values are based on samples ranging in number from 10 to 172. The pyrite content is usually below 1% for sand and several per cent for clay and loam. However, extreme pyrite contents of around 10% may also be encountered in clay from the Naaldwijk Formation. These outliers are for the Wormer Member of the Naaldwijk Formation in Zeeland associated with organic-rich loam immediately below peat (Keijzer & Griffioen, Reference Keijzer and Griffioen2012). The calculated reactive Fe content that is not associated with pyrite is higher in the Neogene Breda and Oosterhout Formations than in the Quaternary Eem and Naaldwijk Formations. The total amount of reactive Fe is thus also higher in these Neogene formations, which implies larger reaction capacities in redox processes (Huisman & Kiden, Reference Huisman and Kiden1998; Keijzer & Griffioen, Reference Keijzer and Griffioen2012). The presence of reactive Fe minerals other than pyrite may thus be considerable and differs among geological formations. No other quantitative information exists on the occurrence of these reactive Fe minerals. However, some qualitative information is available on the occurrence of siderite and glauconite in marine sediments, as discussed below. It is also unknown whether the presence of reactive Fe can be attributed to Fe oxides that are not accessible for reductive dissolution of Fe oxides, as concluded from depth profiles of pyritisation in shallow recent marine sediments (Oenema, Reference Oenema1990a).
Statistically significant differences between sands of the Wormer and Walcheren Members have been found in the cumulative frequency distribution of both pyrite and non-pyrite reactive Fe for the Naaldwijk Formation in Zeeland (Vermooten et al., Reference Vermooten, Griffioen, Heerdink and Visser2011). Similar differences were observed for the loam and clay groups, but this was not tested on significance because the data groups were too small. The two members have a comparable sedimentary genesis (De Mulder et al., Reference De Mulder, Geluk, Ritsema, Westerhoff and Wong2003). Compared with the Wormer Member, the Walcheren Member has substantially lower pyrite contents, lower contents of sedimentary organic matter and higher contents of non-pyrite reactive Fe. The Walcheren Member is younger and the degree of pyritisation is apparently less in these younger sediments. By contrast, no statistically significant differences in cumulative frequency distribution of these three variables were found between the Naaldwijk and Eem Formations in the Northern Netherlands (Klein & Griffioen, Reference Klein and Griffioen2012). Comparison between the data for marine and fluvial or other sediments (Tables 8 and 26) revealed that the marine formations have higher pyrite contents, which may or may not be statistically significant (Vermooten et al., Reference Vermooten, Griffioen, Heerdink and Visser2011; Klein & Griffioen, Reference Klein and Griffioen2012). The non-pyrite reactive Fe contents are comparable, but no significant differences were found.
Two drillings deeper than 60 m in North Brabant revealed that the Breda, Maassluis and Oosterhout Formations contain less than 0.5% S with sporadic isolated peaks up to around 1%, which are often related to organic-rich layers (Huisman & Kiden, Reference Huisman and Kiden1998). These peaks often do not coincide with the total Fe peaks. This leaves open the question of what the speciation is of the reactive Fe and whether sufficient reactive Fe is present to bind all S as pyrite or elemental S. For sand samples from the Neogene Oosterhout and Breda Formations, Hartog et al. (Reference Hartog, Griffioen and Van Bergen2005) found that oxygen consumption during incubation experiments must be attributed to both pyrite and sedimentary organic matter oxidation. The samples contained only 0.1–0.2% S. Laenen & De Craen (Reference Laenen and De Craen2004) report pyrite contents of 0.7–1.0%, with one extreme of 3.2% for one stratigraphical horizon of the Boom Clay in Belgium that contains siderite. This horizon was studied in Belgium and it is unknown whether this stratigraphical horizon occurs in the Netherlands and, if so, how far northwards. The Boom Clay is also present in the Netherlands (stratigraphically classified as the near-equivalent Rupel Clay; De Mulder et al., Reference De Mulder, Geluk, Ritsema, Westerhoff and Wong2003) and is a potential host rock for the disposal of high-level radioactive waste.
5.5.3 Micromorphology of pyrite and Fe oxides
Kooistra (Reference Kooistra1978, Reference Salomons and Mook1981) micromorphologically characterised the redoximorphic features of recent sediments in the Eastern Scheldt and related these diagenetic features to the pedogenic development at the plot scale. Three major occurrences for framboidal pyrite were distinguished: (1) as individual spheres, (2) as amoeboidal pyrite and (3) as pseudomorphs in root remains, peat fragments and detrital grains. Amoeboidal pyrite is described as aggregates of framboids in which smaller framboids are joined to a large framboid or interconnect two larger framboids. A fourth, minor type was idiomorphic, most probably cubic, having diameters less than 25 μm. It was rare, found only in high-lying salt marshes. A range of transitions from a few pyrite framboids to complete occupation with framboids was found in identical sedimentary peat fragments with black components, which indicates that organic matter serves as substrate for sulphate reduction. It is nowadays recognised that framboids form under high supersaturation, so that the nucleation rate is greater than the crystal growth (Ohfuji & Rickard, Reference Ohfuji and Rickard2005). They form when aqueous FeS clusters react via the H2S or polysulphide mechanism, and not directly from solid mackinawite or greigite as was long assumed (Rickard & Morse, Reference Rickard and Morse2005).
The diameters of the individual microlites in the framboids range from 0.5 to 1.5 μm. Pyrite spheres (diameter <12–25 μm) were also found in suspended matter from tidal streams and fresh sediments associated with organic-rich mud flakes and in peat and detritus grains. This implies that pyrite may become eroded, transported and locally deposited, i.e. not all pyrite is thus formed strictly in situ. The framboidal spheres are most common in the recent marine sediments. Amorphous Fe sulphides are present between the spheres. Clusters of pyrite spheres were observed in organic matter and in clay sediment, where much fine-grained organic matter was present. Amoeboidal pyrite consists of spheres with diameters between 20 and 80 μm that represent growing phases, being found in root remains and clay sediments in the reduced zone.
Black components were also observed to be particularly associated with organic material. Energy dispersive X-ray analysis indicated that these may be amorphous Fe sulphides or amorphous Mn oxides. Red ferric iron accumulations occurred in the intertidal and supratidal sediments as coatings or altered coatings around pyrite accumulations and associated with Mn accumulations. Phosphorus was also present in these accumulations, often in association with Ca. They occurred where aeration was possible, which implies that they are found in specific zones. They were composed of amorphous Fe hydroxide or goethite and found in the intertidal flats, natural levees and basins of the middle-high and high salt marshes.
The vertical zoning of pyrite framboids, amorphous Fe sulphides and Fe(III) accumulations as neoformations is related to mean high tide level (MHTL). In the lower regions of the intertidal zone, the Fe(III) accumulations started from the surface and in the middle-high and high salt marshes they started below the surface (Kooistra, Reference Kooistra1978, Reference Kooistra and Bisdom1981). The walls of shrinkage cracks in the sandy intertidal flats usually contained ferric iron accumulations and they were best developed near gullies and the highest parts of the slightly undulating flats. No Fe(III) accumulations were found in the low salt marsh basins where sulphides are found from the surface onwards and MHTL is about equal to surface level. Overall, most Fe(III) accumulations are associated with voids instead of being present in the soil matrix, and are particularly related to faunal channels in the middle-high salt marshes. Summarising, the first metre depth of intertidal and supratidal flats is heterogeneous in the mineralogy of reactive Fe minerals and has its own pedogenetic dynamics.
The micromorphology of sulphides in geological marine sediments has only partly been characterised. Van Rossum (Reference Van Rossum1998) observed framboidal pyrite up to 20 μm as well as fine particles up to 30 μm in Calais Deposits (nowadays classified as the Wormer Member of the Naaldwijk Formation). This range lies within the lower range as observed for pyrite in recent sediments and described above. RGD (1989) observed the same in a few samples of the Breda Formation, and also framboidal aggregates up to 2.0 mm long. Octahedral and cubical pyrite has been observed more rarely in the Breda Formation. Microframboids around 1 μm are found both dispersed and related to carbonate concretions in the Boom Clay; normal framboids of 5–20 μm and large ones up to 100 μm have also been observed in the concretions (Baeyens et al., Reference Baeyens, Maes and Cremers1985; De Craen et al., Reference De Craen, Swennen, Keppens, Macaulay and Kiriakoulakis1999b). Additionally, pyrite is also common in the Boom Clay concretions as octahedral crystals up to 8 μm and as large (< 500 μm) octahedral to cubohedral crystals as cement in the septarian fractures. All these observations confirm that framboids remain preserved over long periods (Wilkin et al., Reference Wilkin, Barnes and Brantley1996).
Microscopic investigation of four glauconite-rich sand samples of the Breda Formation in Zeeland revealed that in addition to pyrite grains, amorphous Fe hydroxide and goethite are present together with secondary Ca carbonate (Schoute et al., Reference Schoute, Bisdom, Schoonderbeek, Boersma, Schreiber and Vos1992). These precipitates cement the primary grains and mostly appear in association with weathered glauconite grains. The samples taken at depths of 25–42 m probably originate from anaerobic conditions and in this context the presence of amorphous Fe hydroxides is remarkable.
5.5.4 Sulphidisation of recent sediments
Sulphidisation and sulphide oxidation in marine sediments and soils were first studied over 150 years ago (Van Bemmelen, Reference Van Bemmelen1863). The initial motivation was the forming of acid sulphate soils after reclamation of marine sediments. We will consider two different geographic settings: recent sediments close to the surface where early diagenesis under natural conditions happens and reclaimed marine soils where pedogenic processes happen.
Sulphidisation of marine sediments has been studied for an intertidal flat in the Wadden Sea (Vosjan & Olanczuk-Neyman, Reference Vosjan and Olanczuk-Neyman1977), salt marshes of the Scheldt estuaries (Oenema, Reference Oenema1990a; Zwolsman et al., Reference Zwolsman, Berger and Van Eck1993), 300–400-year-old sediment in the Eastern Scheldt (Oenema, Reference Oenema1988) as well as in fine-grained, recent sediments from abandoned channels and mussel culture areas in the Eastern Scheldt (Oenema, Reference Oenema1990b). Vosjan & Olanczuk-Neyman (Reference Vosjan and Olanczuk-Neyman1977) observed SO4 reduction in a sandy intertidal flat and dissolved sulphur concentrations of 100–300 μg S/gsed within 25 cm depth, which is contrary to Kooistra's (Reference Kooistra1978) findings that pyritisation is negligible in sandy intertidal flats. However, Vosjan & Olanczuk-Neyman studied only one core.
In the Eastern Scheldt, Oenema (Reference Oenema1990b) analysed both sediment and pore water sampled where fresh faeces rich in organic carbon are produced at mussel plots and rapidly accreting channels. He found that at both types of sites sulphidisation of easily extractable Fe(III) was nearly complete within several tens of centimetres depth. The SO4 reduction rate was also near-identical, averaging 7–70 mmol SO4/(m2.day), with the highest rates observed in summer with highest rate of sedimentation and SO4 becoming completely consumed within 40 cm depth being followed by methanogenesis. The pyrite contents did not vary much with depth, which suggests that Fe monosulphides are produced. The AVS contents varied between 40 and 80 μmol S/cm3, which is somewhat higher than the values found in recent fluvial sediments (Table 14). Note that a dissolved H2S concentration of 1 mmol/L is equivalent to 2 μmol S/cm3 when porosity is 0.5. Such an amount is not uncommon in pore water in estuarine supratidal flats (Oenema, Reference Oenema1990a) and cannot be ignored when AVS is low. Oenema (Reference Oenema1988) also studied early diagenesis in 400-year-old clayey deposits exposed on an intertidal flat. Pyrite content varied between 0.4% and 1.1%, and organic C varied between 1.4% and 2.3%, both invariant with depth within profiles 90 cm deep. The calculated SO4 reduction rate was about 0.4 mmol SO4/(m2.day) in summer and about 0.1 mmol SO4/(m2.day) in winter. However, the CO2 produced was stoichiometrically larger than the SO4 decrease, which suggests oxidation of sulphides in the rhizosphere and underestimation of the true SO4 reduction rate. These rates are substantially lower than those within recent marine sediments.
Oenema (Reference Oenema1990a) explained the pyrite distributions in vegetated salt marshes and natural levees as the result of the simultaneous occurrence of three processes: (1) sedimentation of pyrite-containing material, (2) pyrite oxidation in the upper rooting zone and (3) pyrite formation at the transition from the suboxic to the anoxic zones in a restricted zone 5–10 cm thick. The net rate of pyrite formation below the redox transition equals 7–10 mmol S/(m2.y) in the medium-high marsh. Oenema's electron microscope observations confirmed the more extensive micromorphological observations of Kooistra (Reference Kooistra1978, Reference Kooistra and Bisdom1981). Based on pore water analysis, pyrite oxidation was most intense in the late summer when SO4 at more than double the seawater concentration was observed, Fe concentration was as high as 150 mg/L and pH as low as 2.7–3.5. Pyrite oxidation is negligible in low salt marshes because of near-permanently wet conditions at the top of the sediment. The degree of pyritisation increased with depth from <30% in the oxidised surface layers to 70–80% in the reducing layers. Some residual oxalate-extractable Fe in these reducing layers seems to be not available; this was also noted by Canavan et al. (Reference Canavan, Slomp, Jourabchi, Van Cappellen, Laverman and Van den Berg2006) for freshwater sediments in Haringvliet (see before). The situation is different for unvegetated marshes. Here, no pyrite is produced but pyrite and AVS depth profiles indicate that Fe monosulphides are produced: the dissolved sulphide concentrations were 10–20 times higher than at the vegetated sites. The difference between these two situations is attributed to lack of production of elemental sulphur, which is needed to convert Fe monosulphide to pyrite, and also to small differences in pore water pH. Zwolsman et al. (Reference Zwolsman, Berger and Van Eck1993) confirmed the findings of Oenema (Reference Oenema1990a) in broad lines: they found a decrease in S content within a few centimetres’ depth (which indicates oxidation of recently deposited pyrite) and below 15 or 40 cm depth an increase in S content (indicating in situ production of Fe sulphides). Total Fe does not match the characteristic S profiles and sulphide Fe was not analysed separately.
5.5.5 Pyrite oxidation in reclaimed soils
Micromorphologically, pyrite oxidation products in marine soils of droogmakerij polders (polders reclaimed by draining an open body of water such as a lake or shallow part of the sea) have been characterised in detail by Van Dam & Pons (Reference Van Dam and Pons1972) and Miedema et al. (Reference Miedema, Jongmans and Slager1973). Air as oxidising reactant enters the clay-rich soil primarily via channels and root holes, which explains some of the appearances of the oxidation products. A distinction needs to be drawn between soils that have excess carbonate and remain neutral, and acid sulphate soils where the amount of Ca carbonate is insufficient to buffer the acid production due to oxidation of pyrite with oxygen. For the carbonate-rich situation, amorphous Fe hydroxides occur along large channels as clusters of spheres that are the same size as the original pyrite framboids or as newly formed accumulations. If pyrite oxidation is incomplete, a nucleus of pyrite is found within the Fe hydroxide sphere. The former are attributed to rapid oxidation in more open conditions and the latter are attributed to slower oxidation in which dissolved Fe(II) can move away from the pyrite clusters and become oxidised on the walls of a crack. Associated gypsum is mainly formed in root holes and cracks as result of evaporation and supersaturation.
For acid sulphate soils, three stages or zones have been distinguished, with amorphous Fe hydroxide and jarosite (KFe(III)3(SO4)2(OH)6) as primary products and goethite and gypsum as minor products. The most important oxidation product in the first stage is amorphous Fe hydroxide: it occurs in the lower part of the oxidised zone. The pH is not yet acid and Fe hydroxide is more stable than jarosite. It occurs under the same two conditions as in the calcareous soils and also in Fe-impregnated reed remains. Some jarosite may occur as jarosite framboids or newly formed accumulations. In the second stage, jarosite is dominant mainly along large channels and occasionally as framboids. Amorphous Fe hydroxides have been found to occur regularly as accumulations and Fe-impregnated reed remains, in greater amounts than in stage 1. In this case, the transformation to jarosite is attributed to a slow fall in pH, where SO4 in jarosite still originates from pyrite oxidation and K is omnipresent in soils. Otherwise, jarosite production under pyrite oxidation is instantaneous when oxygen enters relatively quickly, pH falls rapidly and jarosite occurs as pseudomorphic spheres. In the third stage and uppermost part of the soil, amorphous Fe hydroxides mainly occur – in even larger amounts than in stage 2. They are mainly present as accumulations and as Fe-impregnated reed remains. Some jarosite has also been observed in similar forms as stage 2, but it was more strongly stained by Fe hydroxides. In this stage, protons have been leached, pH is higher than in zone 2 and jarosite has been hydrolised to Fe hydroxide. Iron(III) is more mobile under the acidic conditions and Fe hydroxide is formed as accumulations. Some goethite may be formed mainly in this zone, in the largest channels which dry out most during dry periods. It is worth mentioning that under the prevailing acid conditions some clay minerals become unstable and start to hydrolyse. During this weathering process cryptocrystalline silica may be formed.
5.5.6 Glauconite
Glauconite is mineralogically related to the clay minerals but unlike the other clay minerals its origin is primarily autochthonous and not detrital in Dutch sediment. It is therefore discussed separately together with other reactive Fe minerals. Glauconite is diagenetically formed in marine environments; Chafetz & Reid (Reference Chafetz and Reid2000) realised that it may also form in shallow marine conditions. It gives a characteristic green colour to the sediments (Fig. 14).

Fig. 14. Example of glauconite-rich sand illustrating the typical occurrence of this mica or clay mineral as sand-sized granules.
The Neogene marine Breda Formation is mostly glauconite-rich, having a green or green-black colour: generally, De Mulder et al. (Reference De Mulder, Geluk, Ritsema, Westerhoff and Wong2003) speak of glauconite contents up to 50%, specifically, Schoute et al. (Reference Schoute, Bisdom, Schoonderbeek, Boersma, Schreiber and Vos1992) report about 5–20% volume with average grain size of 84–181 μm for just four samples taken from a borehole in the Breda Formation in Zeeland. Based on K/Ar glauconite dating, Vandenberghe et al. (Reference Vandenberghe, Harris, Wampler, Houthuys, Louwye, Adriaens, Vos, Lanckacker, Matthijs, Deckers, Verhaegen, Laga, Westerhoff and Munsterman2014) showed for the Belgian equivalent of the Breda Formation that the highest glauconite contents may be due to in situ production whereas lower contents in younger sediments are due to reworking of these layers and mixing with sediments from the hinterland. Similar patterns are likely for the Dutch Breda Formation and other glauconite-bearing deposits. In three stratigraphical Breda members that occur in South Limburg glauconite is present in small amounts or is absent; the two phosphorite-bearing members that occur in the eastern Netherlands are also rich in glauconite (De Mulder et al., Reference De Mulder, Geluk, Ritsema, Westerhoff and Wong2003). The Pliocene marine Oosterhout Formation is glauconite-poor (albeit that it may be locally rich in glauconite; www.dinoloket.nl); this and its higher shell content distinguish it from the underlying Breda Formation. The Early Pleistocene marine Maassluis Formation may be locally poor in glauconite. When this is the case, it is difficult to distinguish from the Oosterhout Formation. It is worth pointing out that suspended matter of the Scheldt also contains a little glauconite because of erosion of Neogene/Palaeogene deposits (Wartel, Reference Wartel1977; Baeyens et al., Reference Baeyens, Van Eck, Lambert, Wollast and Goeyens1998). This is thus detrital glauconite, which suggests that recent sediments from the River Scheldt may contain some glauconite.
The presence of glauconite in marine sand can be chemically recognised by K2O:Al2O3 ratios that are higher than in glauconite-free sand (Huisman & Kiden, Reference Huisman and Kiden1998). Unfortunately, more direct analytical evidence for glauconite contents is lacking. Fraters et al. (Reference Fraters, Boumans, Van Elzakker, Gast, Griffioen, Klaver, Nelemans, Veld and Velthof2006) characterised glauconite-rich samples from the Eocene marine Dongen Formation on reactive Fe. They found 1–3% reactive Fe in mostly clay sediments, which suggests that glauconite may be part of the reactive Fe as defined earlier. Several studies also reveal that Fe structurally bound in silicates is redox-sensitive too (Fanning et al., Reference Fanning, Rabenhorst, May and Wagner1989; Ernsten et al., Reference Ernsten, Gates and Stucki1998; Kostka et al., Reference Kotska, Wu, Nealson and Stucki1999; Rivaz Perez et al., Reference Rivaz Perez, Banwart and Puigdomenech2005), i.e. glauconite may be part of the sediment oxidation/reduction capacity.
5.5.7 Fe carbonate
De Craen et al. (Reference De Craen, Swennen and Keppens1999a,b) and Laenen & De Craen (Reference Laenen and De Craen2004) described the occurrence of ferroan calcite and siderite, respectively, in carbonate concretions in the Belgian Boom Clay (which is near-equivalent to the Rupel Clay in the Netherlands). For ferroan calcite, two sediment-petrological phenomena were described. First, the concretions themselves showed increasing Fe content from the centre to the rim, with Fe content increasing from 4000 ppm to somewhat more than 6000 ppm. The increase in Fe and partly also in Mn reflects a shift towards increasingly reducing conditions during diagenetic growth. Septarian cracks within the concretions were also lined with several generations of fibrous calcite cement. The third and fourth generations were composed of ferroan calcite instead of calcite. The isotopic evolution for δ13C and δ18O of this cement indicates progressive precipitation from sulphate reduction to methanogenesis. These septarian concretions occur in a series of horizons throughout the Boom Clay.
The second phenomenon is siderite in carbonate concretions from one particular stratigraphical horizon within Boom Clay (or two; De Craen et al., Reference De Craen, Swennen, Keppens, Macaulay and Kiriakoulakis1999b), where it accounts for on average 15% of the total carbonate content. The explanation for its genesis is a unique sedimentary sequence, where during a period of slow sedimentation Fe oxides could concentrate in the aerobic zone of the sediment. Subsequently, the high rate of reductive dissolution of these Fe oxides could not be matched by the rate of sulphide production under anaerobic conditions during a period of high sedimentation. As a result, siderite became formed together with framboidal pyrite. Manganese and phosphate are also enriched in this horizon, which further supports the explanation.
5.6 Solid organic matter
5.6.1 Organic matter contents and carbon isotopes
Table 26 presents statistical data on organic carbon contents for a variety of marine geological formations in different regions. As expected, the contents are higher in loam and clay than in sand. The contents in sands typically range up to about 2% and in loam and clay up to 4% or 5%. The contrast between the Holocene and older formations is not as clear as for the fluvial formations (section 4.6). The organic carbon contents in suspended matter in marine or estuarine environments are higher, with ranges typically from 3% or 5% to 10% or 20% (Table 16). The contents in recent estuarine sediments are several per cent but are lower for recent marine sediments. Suspended matter is thus richest in organic carbon. Wiesner et al. (Reference Wiesner, Haake and Wirth1990) noted a positive correlation between the total organic carbon content of marine sediments in the central North Sea and the weight percentage of the fine fractions (smaller than 20 μm). They noted a ‘background’ organic carbon content per size class ranging from about 3.4% for the <2 μm class to about 0.3% for the 20–63 μm class. Higher contents may occur, which are related to settling of locally produced organic matter and mixing of sediment due to bioturbation or erosion/deposition cycles.
The isotopic behaviour of bulk OM in recent sediment and suspended matter of the North Sea and the estuaries of the Rhine, Scheldt and Ems has been characterised in a series of publications (Salomons & Mook, Reference Salomons and Mook1981; Laane et al., Reference Laane, Turkstra, Mook, Ittekot, Kempe, Michaelis and Spitzy1990; Dauwe & Middelburg, Reference Dauwe and Middelburg1998; Abril et al., Reference Abril, Nogueira, Etcheber, Cabecadas, Lemaire and Brogueira2002; Middelburg & Herman, Reference Middelburg and Herman2007; specifically for the Scheldt also Mariotti et al., Reference Mariotti, Lancelot and Billen1984; Middelburg & Nieuwenhuize, Reference Middelburg and Nieuwenhuize1998; Hellings et al., Reference Hellings, Dehairs, Tackx, Keppens and Baeyens1999; De Brabandere et al., Reference De Brabandere, Dehairs, Van Damme, Brion, Meire and Daro2002; Chen et al., Reference Chen, Wartel, Van Eck and Van Maldegem2005). Organic matter in coastal and estuarine systems may originate from several sources, each of which has a different carbon isotope signature (Middelburg & Herman, Reference Middelburg and Herman2007): marine organic matter (δ13C from –18 to –22‰), riverine algae (δ13C –30 to –40‰), terrestrial soil organic matter (δ13C –26 to –27‰) and sewage (δ13C –14 to –28‰).
The isotopic composition of organic carbon in recent coastal marine sediment and suspended matter was studied by Salomons & Mook (Reference Salomons and Mook1981), Laane et al. (Reference Laane, Turkstra, Mook, Ittekot, Kempe, Michaelis and Spitzy1990) and Dauwe & Middelburg (Reference Dauwe and Middelburg1998). The data show a range in values from –25‰ to –20‰, with the most negative values close to the mouth of the Nieuwe Waterweg and the Scheldt or at the Wadden Sea and an incidental measurement of about –16‰ for suspended or sedimentary organic matter. The most negative values are equivalent to those of organic matter in the Dutch fluvial systems (Table 16). This shows that fractions of continental organic matter are present in the Dutch coastal environment. The 14C values also refer to aged organic matter, where it is interesting to note that the highest 14C value corresponds to the least negative δ13C value. The marine-derived organic matter present is thus apparently younger than the organic matter derived from the continent. Chen et al. (Reference Chen, Wartel, Van Eck and Van Maldegem2005) report δ13C values of –22 to –21‰ for suspended organic matter in front of the mouth of the Scheldt, which indicates a marine origin.
The δ13C values of sedimentary OM in a homogeneous series that also contained several Neogene marine samples averaged –25.1 ‰ ± 1.1 irrespective of geological formation or depth of burial (Table 16; Hartog et al., Reference Hartog, Griffioen and Van Bergen2005). Such values refer to a dominance of terrestrially derived OM. For comparison, Römkens et al. (Reference Römkens, Van der Plicht and Hassink1999) found –27.7 to –25.9‰ as δ13C values for OM in sandy soil samples at a farm in North Brabant. The values were higher with increased contribution of maize and varied with individual grain size class, depth and bulk density. Relatedly, the 14C values were close to 100 PMC for the topsoil, whereas for the subsoil at 60–80 cm depth they were 74.7 PMC below pasture and 82.4 below maize (Römkens et al., Reference Römkens, Hassink and Van der Plicht1998). The values also varied with grain size class and bulk density for the two topsoil samples.
5.6.2 Organic matter in estuaries
The physical differences between the three estuaries of Rhine, Ems and Scheldt (see Fig. 1) influence the behaviour of particulate organic matter (Table 27; Abril et al., Reference Abril, Nogueira, Etcheber, Cabecadas, Lemaire and Brogueira2002). The Rhine estuary is a river-dominated estuary, with relatively high river discharges, short residence time, stratified vertical salinity profile and an extended plume at sea. Its inner area is heavily impacted by hydraulic engineering and is strongly reduced in size. The Ems and Scheldt are well-mixed turbid estuaries with small river discharge. The Scheldt has a long residence time and the Ems has an intermediate residence time. The Scheldt is also severely polluted with organic waste, as until recently there were no wastewater treatment facilities in large parts of the catchment. The Scheldt River system can be separated into three parts: (1) the fresh part upstream of Ghent (which is beyond the scope of this review), (2) the Belgian Zeeschelde up to Ghent (57.5–155 km from the mouth at Vlissingen), which is tidal and has increasingly brackish water downstream from Rupelmonde (92 km) and (3) the Dutch Western Scheldt (0–5.7 km), which has saline water. The maximum turbidity zone extends roughly from 90 to 110 km from the mouth, where the turbidity is highest due to tidal forcing.
Table 27. Characteristics of the recent estuaries related to the Netherlands (derived from Abril et al. (Reference Abril, Nogueira, Etcheber, Cabecadas, Lemaire and Brogueira2002)).
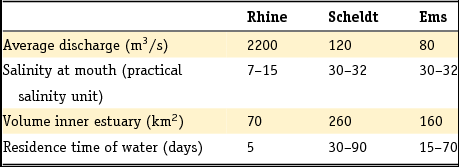
A common characteristic of the three estuaries is that the concentration of POC (particulate organic carbon) decreases with salinity, although the maximum values for the Rhine (about 1 mg/L) are several factors lower than for the other two. This is mainly due to the Rhine having a more natural decontaminated status in the monitored period at the end of the 1990s. The Rhine estuary has about 5% organic carbon in its suspended matter for the inland low saline zone (0–20 PSU) and 5–20% for its high saline zone, which extends out to sea from the coastline, where the highest contents are observed in spring/summer (Abril et al., Reference Abril, Nogueira, Etcheber, Cabecadas, Lemaire and Brogueira2002; Middelburg & Herman, Reference Middelburg and Herman2007). The δ13C values of suspended organic carbon increase with salinity from –27/–26‰ to –24/–20‰, showing an increase in marine signature (Table 16). The δ13C results for suspended matter sampled in 1990 are comparable to those for sediment sampled in 1957/58 from the Haringvliet and the Grevelingen before closure by the Delta works. These are all indications of the stable nature of POC in the inland part of the Rhine estuary. Algal POC is substantial only in the most saline part.
The suspended matter in the Ems estuary has rather low and constant organic carbon contents (3–5%), with lowest values at intermediate salinity and a rather flat profile versus salinity for δ13C (–24‰ to –22‰). Algal POC is only relevant in summer at salinities above 25 PSU. The δ13C for its estuarine sediment is somewhat lower, suggesting a more terrestrial origin; the values for the fresh part are even more negative. The Ems estuary is a turbid estuary in which tidal mixing flattens any organic carbon gradient between the fluvial and marine end-members. Abril et al. (Reference Abril, Nogueira, Etcheber, Cabecadas, Lemaire and Brogueira2002) deduced that about 50% of fluvially imported POC, probably from anthropogenic pollution, is mineralised in the estuary.
The situation for the Scheldt is different for the period in the 1990s studied because of the severe pollution with sewerage-derived material. It is far from representative for the Scheldt system during geological time. Suspended POC in the fluvial stretch is about 18.3 mg/L, decreasing sharply to a few milligrams per litre at salinities of 15 PSU and above. The OC content of suspended matter varies between 3% and 20%, with the highest percentages found at low salinity and a range between 17 and 27 PSU. The highest percentages are partly attributable to autochthonous organic matter production in spring and summer, as evidenced by the seasonal fluctuation in δ15N values of suspended matter (Mariotti et al., Reference Mariotti, Lancelot and Billen1984). The δ13C values increase near-linearly with salinity, from –28/–30‰ to –19/–23‰. Here, a seasonal fluctuation is observed, with most negative values in the fresh stretch of the Zeeschelde, which indicates a contribution of fluvial algae or its detritus (also confirmed by temporal fluctuations in δ15N values). The algal POC is lowest in the maximum turbidity zone, where light penetration is least. A large loss of continental POC thus occurs along the Scheldt estuary, with most mineralisation occurring in the Belgian Zeeschelde. Although the Scheldt estuary is considered to be a well-mixed estuary, its behaviour is intermediate between that of the Rhine and the Ems estuaries. The OC content has not levelled off at the mouth, which implies that mineralisation of Scheldt-derived POC probably continues under marine conditions. In a single sampling campaign in August, Middelburg & Nieuwenhuize (Reference Middelburg and Nieuwenhuize1998) found that the sedimentary organic matter differs from suspended organic matter in its δ13C and also C/N ratio and δ15N. Sediment in the upper Zeeschelde had an average δ13C of –26.3‰ ± 0.5 and the lower Western Scheldt –23.5‰ ± 1.0; the corresponding values for suspended matter were –28.9‰ ± 1.1 and –20.1‰ ± 1.7. Boschker et al. (Reference Boschker, Brouwer and Cappenberg1999) observed comparable values from –25.7 ± 0.8‰ to –22.2 ± 1.1‰ depending on whether the flat was vegetated or bare. The sediment thus has a smaller range and appears to be more dominated by soil organic matter, whereas the suspended matter is more a variable mixture of fresh and estuarine algal matter and marine organic matter. Laane et al. (Reference Laane, Turkstra, Mook, Ittekot, Kempe, Michaelis and Spitzy1990) reported a similar average for sediment in the Western Scheldt but a more negative average (–26.6‰ ± 2.2) for suspended matter. The algal matter will be less important in autumn and winter, which may more strongly change the isotopic value for suspended organic matter. Rather different values were observed by Boschker et al. (Reference Boschker, Brouwer and Cappenberg1999) for a flat in the Eastern Scheldt: values around –20‰ irrespective of whether or not the site was vegetated. The difference between the two estuaries was attributed to differences in sedimentation rate and related burial of algal and non-algal organic matter.
5.6.3 Organic matter in the southern North Sea
The organic geochemistry of suspended matter and recent sediments in the North Sea has been studied using various analytical techniques. Boon & Duineveld (Reference Boon and Duineveld1996) studied near-bottom suspended matter at four stations along the coast from the Netherlands to the Skagerrak, whereas Dauwe & Middelburg (Reference Dauwe and Middelburg1998) studied shallow sediments at these four stations as well as two additional ones. Lardinois et al. (Reference Lardinois, Eisma and Chen1995) studied suspended matter at about 30 sites across the North Sea, including those studied by the former two. Wiesner et al. (Reference Wiesner, Haake and Wirth1990) studied recent sediments in the central North Sea.
Lardinois et al. (Reference Lardinois, Eisma and Chen1995) characterised the lipid, protein and chitin contents of suspended organic matter in the North Sea for the winter half year in which no large concentrations of phytoplankton occur. The proteins were by far most abundant, with around 70–85%, the lipids represented 13–26% and the chitin only 1–3%. As a result, the functional composition of suspended organic matter was very similar to that of phytoplankton, assuming that a stoichiometrically equivalent amount of carbohydrates is present as well. The discrepancy can be attributed to a small contribution of zooplankton, bacterial and terrestrially derived OM. The functional group analysis is in general harmony with the finding of the carbon isotope analysis that marine-derived organic matter is dominant in marine suspended organic matter.
Wiesner et al. (Reference Wiesner, Haake and Wirth1990) and Dauwe & Middelburg (Reference Dauwe and Middelburg1998) both studied shallow sediments. The former focused on the more stable compounds and the latter on the amino acids as markers for biodegradation. Wiesner et al. found that the fraction of terrigeneous organic carbon in terms of total organic carbon was 68 ± 7% for mud, 83 ± 13% for heteroclastic sediment and 84 ± 10% for sand. This fraction is dominated by vitrinite (the glass-like coalified component of coal) and inertinite (the charred component of coal). Both are derived from the ligno-cellulose components of higher plants (Engel & Macko, Reference Engel and Macko1993). There is thus a larger fraction of chemically resistant components in the coarse sediments. The alginite and liptodetrinite compounds can also both be substantial. Alganite is the fossilised and coalified product of marine algae and is more strongly restricted to mud. The aliphatic hydrocarbons that were analysed for a small subset of samples point to an admixture of relatively unaltered algal lipids in the fine fraction irrespective of the lithological class of the sample. The general differences between sand and clay can be attributed to sand being more exposed to oxygen. Wiesner et al. (Reference Wiesner, Haake and Wirth1990) attributed the amount and state of organic matter in the sediment to a combination of three factors: (1) the availability of locally produced organic matter, (2) the concentration and size distribution of suspended matter in the overlying water column and (3) the duration of periods of reduced current velocity versus turbulence. Here, a high local primary production may give rise to increased OM contents in sand as well as mud, but the difference from the background situation will be more marked for sand.
Dauwe & Middelburg (Reference Dauwe and Middelburg1998) determined that amino acids and carbohydrates each composed 8–28% of the total organic carbon, amino sugars accounted for only about 2% and the rest remained unidentified. The identified fraction of OM was highest for the muddy or silty sediments. The δ13C of the organic material in the upper 0.5 cm had values typical for marine organic matter (Table 16), with slightly more negative values for the deposition areas of the North Sea (Skagerrak and German Bight). The values are somewhat low when compared with the findings of Wiesner et al. (Reference Wiesner, Haake and Wirth1990), who observed that most organic matter in North Sea sediments is terrigeneous instead of marine. A principal component analysis on the amino acids group showed systematic differences, particularly for neutral protein amino acids and non-protein amino acids, due to selective degradation with an overall quality range from source-like material to refractory. The differences were thought to be not only due to in situ degradation but also to degradation prior to deposition. The sandy sediments in the southern North Sea had the freshest organic matter, whereas organic matter in the muddy sediment at the Skagerrak had been degraded most extensively. The non-protein amino acids were highest for the Skagerrak sediment because they had not been incorporated into biota. Note that amino acids occur in various marine and terrestrial organisms and no amino acid is unique to any source (Cowie & Hedges, Reference Cowie and Hedges1992). The two amino sugars are relatively resistant to degradation because they are incorporated into structural biopolymer matrices as chitinous material. They were indeed most concentrated in the refractory sediments.
Phytopigments and fatty acids in near-bottom suspended matter were studied by Boon & Duineveld (Reference Boon and Duineveld1996) for four stations ranging from off the coast of Texel to the Skagerrak, in order to ascertain the effect of resuspension of sediment on the composition of suspended organic matter. The analyses indicated that the geographical differences were more significant than the seasonal variations. Total fatty acids and total chlorophyll-a were strongly correlated, illustrating that the fatty acids were mainly derived from algal material. The station off the coast of Texel showed the freshest, least-degraded suspended organic matter with the fewest terrestrial markers. This station can be considered as part of the southern North Sea, having a coarse sand bottom, shallow depth and limited resuspension due to coarse grain size. At the Skagerrak, the algal part is most degraded. Because resuspension does not play an important role here, due to the Skagerrak's depth, the terrestrial markers were low. The two intermediate stations showed the most complex behaviour: suspended organic matter appeared to be more degraded due to the resuspension of refractory organic matter, which was partly terrigeneous.
5.6.4 Organic matter in Paleogene and Neogene sediments
Hartog et al. (Reference Hartog, Van Bergen, De Leeuw and Griffioen2004, Reference Hartog, Griffioen and Van Bergen2005) analysed two series of Neogene marine sand samples together with other samples on molecular composition of organic matter, using pyrolysis-GC-MS. The general results are described in section 4.6 and the specific results for the marine Breda and Oosterhout Formations are summarised here. As mentioned earlier, the marine sediment samples contained terrestrially derived OM and no explicit marine-derived OM. When compared with the samples from the other sedimentary environments, the Neogene marine sediment samples were rich in phenolic and guaicacyl-lignin derived compounds and poor in fatty acids and branched hydrocarbons. A minor contribution of what was probably dead bacterial biomass was also found. There was also a dominance of guaiacyl units with preserved side-chains, from which it can be inferred that the sediment has remained preserved at an early stage of OM degradation. Hartog et al. (Reference Hartog, Van Bergen, De Leeuw and Griffioen2004, Reference Hartog, Griffioen and Van Bergen2005) therefore concluded that these sediments were apparently deposited with limited oxygen exposure time under stable conditions. The initial presence of marine-derived organic matter and the production of pyrite may have preserved the more recalcitrant terrestrially derived OM.
The organic geochemistry of the Belgian Boom Clay was studied by Deniau et al. (Reference Deniau, Derenne, Beaucaire, Pitsch and Largeau2001, Reference Deniau, Derenne, Beaucaire, Pitsch and Largeau2004, Reference Deniau, Behar, Largeau, De Canniere, Beaucaire and Pitsch2005) for a sample from the underground laboratory in Mol. They focused on the kerogen, i.e. the complex, high-molecular-weight organic matter that is insoluble in non-polar organic solvents, alkali and non-oxidising acids. The kerogen has a high aliphatic nature with a substantial amount of N and little S. The bulk of the OM was nanoscopically amorphous without any regular organisation in relation to the clay minerals. The major contribution was from phytoplanktonic material, as evidenced by the presence of, amongst others, degradation products of algal proteins, n-alkanols from green algae and medium-chain, even-carbon-numbered fatty acids. This observation is strikingly different from those of Hartog et al. (Reference Hartog, Van Bergen, De Leeuw and Griffioen2004, Reference Hartog, Griffioen and Van Bergen2005) on Neogene marine sands. Some input of terrestrial and bacterial material was found as, for example, indicated by the presence of long-chain fatty acids and the pyrolysis products of lignin for the Oligocene Boom Clay, and the presence of branched fatty acids, branched hydrocarbons and hopanoid compounds for the Neogene marine sands. As a result, the organic matter in the clay samples is rather immature, with a major role for degradation–recondensation in the formation of the kerogen. Selective preservation, mineral-sorptive protection and natural sulphurisation must have played a minor role.
6 Other sediments
Sediments other than marine or fluvial are present in the subsurface of the Netherlands as well (cf. Fig. 3). Glacial deposits originating from the two glaciations that reached the Netherlands are present in the north of the country. There are also recent and geological lacustrine sediments. The mineralogy of the recent sediment and suspended matter in the large lakes that are associated with the surface water systems of the big rivers were discussed in section 4. Here, we will focus on the remaining types of lacustrine sediments. We will discuss various kinds of aeolian sediments important in the Netherlands: cover sand, loess and coastal dunes. The first two were deposited during the last glaciation. The coastal dunes are Holocene in origin and are active in terms of erosion and accumulation, although their morphodynamics are nowadays managed. Aeolian drift sands are also present in the Netherlands but we are not aware of any mineralogical studies of them. We will discuss peat and bog ore, even though these are not sediments. Peat is an important geomaterial in the Dutch subsurface, especially close to or at the surface. Bog ore (synonymous with bog iron ore) is a special local phenomenon that has been well studied.
6.1 Glacial deposits
Two lithostratigraphical formations with glacial deposits are discerned in the Netherlands: the Peelo Formation from the Elsterian age and the Drente Formation from Saalian. Two well-known deposits in the northern part of the country are the Potklei (Nieuwolda Member) from the first and glacial till or boulder clay (‘keileem’; Gieten Member) from the second. Potklei is actually a glaciolacustrine to marine infilling of subglacial tunnel valleys and is often varved. The boulder clay was deposited as a till during the Saalian glaciation and regionally forms a less than 1–40 m thick layer in the northern and eastern provinces. The glacial sediments have been studied less intensively on their mineralogy than the fluvial and marine sediments. For the Peelo Formation, the clay mineralogy was characterised on the clay fraction of one sandy, aquifer sample (Breeuwsma & Zwijnen Reference Breeuwsma and Zwijnen1984) and on three clay samples from the Potklei (Breeuwsma et al., Reference Breeuwsma, Balkema and Zwijnen1987). The findings for the sand-derived sample differ somewhat from those for the clay samples. Illite is the dominant clay mineral (30–50%), kaolinite (5–30%) and smectite (10–50%) are also important but are more important in the clay samples than in the sand sample, chlorite is low (<5–10%) and no vermiculite or illite chlorite were detected. Glauconite may be found as accessory mineral in both glacial formations, where it occurs locally within the Uitdam Member of the Drente Formation (www.dinoloket.nl). This is the result of the reworking of older marine deposits. Similarly, Pleistocene glacial sediments may contain radiolaria or diatoms from reworked Tertiary sediments (Anderson, Reference Anderson1970; P. Vos, pers. comm.). This makes Crommelin's (Reference Crommelin1945) single observation that Potklei contains amounts of diatoms below the detection limit not representative (Table 25; Crommelin, Reference Crommelin1945), although it will generally hold that glacial sediments contain few diatoms.
The boulder clay is poorly sorted, compact and contains stones and boulders as rock fragments, in addition to grains of individual minerals (De Mulder et al., Reference De Mulder, Geluk, Ritsema, Westerhoff and Wong2003). All kinds of rock fragments can be present: granite, metamorphic, sandstone, limestone (including dolomite) and flint. Two major types of boulder clay were initially distinguished (De Waard, Reference De Waard1949; Zandstra, Reference Zandstra1971, Reference Zandstra and Ehlers1983): (1) grey or common type boulder clay with an average carbonate content of about 7% in the matrix when unweathered, a high flint content, hardly any limestone and a predominance of crystalline rocks of central Scandinavian provenance, and (2) red boulder clay, occurring as masses on and in the grey type, with an average carbonate content of about 24% when unweathered, considerable limestone, little flint and crystalline components of eastern Scandinavian provenance. The distinction by colour is classically made by geologists, where it must be realised that secondary chemical processes may also change the colour. Later on, as many as 11 types of boulder clay were distinguished (Zandstra, Reference Zandstra1974). The mutual differences are explained as a difference in ice flows and provenance, rather than by pedogenesis or diagenesis.
Heavy mineral contents indicate the presence of a garnet-epidote in the grey boulder clay and a hornblende-garnet association in the red boulder clay. Riezebos (Reference Riezebos1968), who characterised individual grain size classes mineralogically, showed that the frequencies of the minerals are influenced by the grain size class. His results indicate that it is impossible to predict which fractions to analyse to obtain the most reliable information. Remarkably, when compared with other Dutch sediments studied, the percentage of heavy minerals in the isolated sand fraction has been determined many times for boulder clay. It is usually 0.2–0.5 in the 125–250 or 40–500 μm fraction, and in only two red boulder clay samples it was 1.0% (De Waard, Reference De Waard1949; Rappol, Reference Rappol1984, Reference Rappol1986). The latter percentage is high for Dutch sediments (Druif, Reference Druif1927).
Both De Waard (Reference De Waard1949) and Riezebos (Reference Riezebos1968) found that the red boulder clay (also referred to a Voorst type) is less rich in quartz and more abundant in feldspars and rock fragments. The feldspars are mainly found in the size class below 150 μm and the rock fragments in the size class above 75 μm (note that grains up to 420 μm were mineralogically investigated). Based on small sample series, Druif (Reference Druif1927), Van Baren (Reference Van Baren1934) and De Waard (Reference De Waard1949) found that the dominant feldspar minerals are orthoclase and/or albite. With respect to feldspars, these glacial sediments are thus more similar to fluvial deposits of Rhine origin than of Eridanos origin (cf. Table 4). Tills may contain considerable amounts of local, preglacial sediments, even occurring as lenses within the till (Rappol, Reference Rappol1984, Reference Rappol1986). Micas are also observed, especially in the size class below 16 μm, where more weathered mica is found in the grey boulder clay than the red boulder clay. The opaque minerals have been found to be dominated by oxides and not pyrite. The amounts of ilmenite, leucoxene and rutile were above 10% of the total amount of grains; magnetite, haematite and also goethite and chromite in the red boulder clay each contributed between 2% and 10%. Druif (Reference Druif1927) observed ilmenite and magnetite as important heavy minerals in all samples and biotite, glauconite and apatite in only a few samples or in only one sample. The identification of biotite is particularly interesting because biotite and mica are both phyllosilicates but biotite weathers considerably faster.
Klein & Griffioen (Reference Klein and Griffioen2010, Reference Klein and Griffioen2012) evaluated carbonate analyses of samples from the Drente and Peelo Formations. They concluded that the Drente Formation as a whole is mostly calcareous, and in the Peelo Formation the sand is mostly poor in carbonate and the clay is rich in carbonate (Tables 9 and 28). Van der Marel (Reference Van der Marel1950) concluded that calcite is negligible or absent in the clay fraction (<2 μm) of soils developed on glacial till or Potklei and that dolomite, siderite and magnesite are not present. This suggests that carbonate is mainly present in the silt fraction, as is the case for fluvial sediments (see section 4.4). Hartog et al. (Reference Hartog, Griffioen and Van Bergen2005) analysed the isotopic composition of carbonate in two samples from the Drente Formation in eastern Netherlands and found values close to 0‰ for both δ13C and δ18O. This implies an allogenic marine origin instead of a syngenetic origin, attributable to reworking of marine sediments from the Oosterhout or Breda Formations or erosion of Fenno-Scandinavian limestone and subsequent glacial transport. The carbonate status of glacial till has received attention in the past, albeit mostly qualitatively, in order to distinguish between the different types of till. As mentioned earlier, the Voorst-type orred boulder clay has an average carbonate content of about 24% in the matrix when unweathered (De Waard, Reference De Waard1949; Rappol, Reference Rappol1986). It then also contains 30–60% limestone as rock fragments (De Waard, Reference De Waard1949; Zandstra, Reference Zandstra1967; Rappol, Reference Rappol1986), which has an East Sea provenance. Other unweathered boulder clay types contain 2–12% carbonate in the matrix and 10–40% limestone and chalk rock fragments (Rappol, Reference Rappol1986). As is the case for marine sediments (see section 5.4.1), the carbonate content is highest in the silt fraction, lowest in the fine- to medium-grained sand fraction and higher again in the coarser sand fraction (Rappol, Reference Rappol1983). The boulder clay is frequently decalcified after deposition and the depth of decalcification depends on the local hydrogeological conditions (De Waard, Reference De Waard1949; Rappol, Reference Rappol1984, Reference Rappol1986). The evidence for decalcification is the similar amount of flint fragments (i.e. similar provenance) in non-calcareous and calcareous boulder clay within individual profiles (De Waard, Reference De Waard1949; Zandstra, Reference Zandstra1967).
Table 28. Regional characteristics (as percentiles) of the pyrite, reactive Fe, organic matter and Ca carbonate contents (in wt%) in the two Dutch glacial formations on the Central Drenthe Plateau (derived from Klein & Griffioen (Reference Klein and Griffioen2012)).

Klein & Griffioen (Reference Klein and Griffioen2012) also present data for pyrite, reactive Fe other than pyrite and organic matter (Table 28): the Drente Formation generally has negligible amounts of pyrite but amounts above 0.1% pyrite can be found and amounts above 1% are rare. Sand of the Peelo Formation is generally poor in pyrite, but amounts between 0.1% and 1% are also found; clay and loam frequently contain pyrite, the average contents being 0.8%. In both formations, the non-pyritic reactive Fe accounts for around 0.5% in all lithological classes. The contrast in sediment Fe chemistry thus seems restricted to that of pyrite. The pattern for organic matter mirrors that of pyrite: low contents in the Drente sediments and sand of the Peelo, and higher contents in its clay and to a minor extent in its loam.
The δ13C values of sedimentary OM in a uniform series of 28 samples that also contained several glacial sediments from the Drente Formation was on average –25.1‰ ± 1.1 irrespective of geological formation or depth of burial (Table 16; Hartog et al., Reference Hartog, Griffioen and Van Bergen2005). Such values testify to a dominance of terrestrially derived OM (see section 5.6). Two samples from the Drente Formation were analysed on molecular composition of organic matter using pyrolysis-GC-MS together with samples from other origins (Hartog et al., Reference Hartog, Van Bergen, De Leeuw and Griffioen2004, Reference Hartog, Griffioen and Van Bergen2005) and the general results are described in section 4.6. When compared with the samples from the other sedimentary environments, the Drente samples were dominated by lignin-derived markers, had a higher degree of side-chain oxidation of hopanoids and lignin derivatives, and contained more macromolecular OM than low-molecular-weight compounds. This indicates that the OM has undergone severe aerobic degradation, as was discussed more extensively in section 5.6. Glacial deposits such as the Drente Formation often contain reworked sediments, and Hartog et al. (Reference Hartog, Van Bergen, De Leeuw and Griffioen2004) suggested that reworking increases the oxygen exposure time, which affects the reactivity of its sedimentary OM.
Gravel- to sand-sized nodules containing vivianite were observed by Riezebos & Rappol (Reference Riezebos and Rappol1987) in Saalian till in Twente, East Netherlands. The nodules were 2–5 mm in diameter and were associated with sedimentary rock fragments of similar size containing glauconite and phosphorites. Vivianite is also associated with carbonates in these rock fragments. The till layer was found on top of the Oligocene marine Rupel Formation. Thin-section studies suggested that the nodules are authigenic (Riezebos & Rappol, Reference Riezebos and Rappol1987). A primary control in the occurrence of such nodules seems to be the presence of phosphate-rich sediments that have been glacially eroded: the probable mechanism is diagenetic conversion of Ca phosphates into vivianite. This mineral can be found in the eastern part of the Netherlands, where Tertiary marine sediments lie close to the surface and are known to contain phosphorite nodules (Laban, Reference Laban1988).
6.2 Lacustrine deposits
No geological formations predominantly composed of lacustrine deposits occur in the Netherlands. Lacustrine deposits are found in the Echteld Formation and also in the Boxtel (as Tilligte Member), Peelo (Potklei as Nieuwolda Member) and Drente (as Uitdam Member) Formations, where they were formed under periglacial conditions (De Mulder et al., Reference De Mulder, Geluk, Ritsema, Westerhoff and Wong2003). However, we know of no studies that have addressed these periglacial deposits individually. Here, we will discuss lacustrine sediments found in small isolated lakes and fens or buried in the glacially excavated Amsterdam basin and of Middle Pleistocene origin.
Marnette et al. (Reference Marnette, Van Breemen, Hordijk and Cappenberg1993) studied the S chemistry of two moorland pools in the Veluwe, looking especially at the Fe sulphides and organic S; Feijtel et al. (Reference Feijtel, Salingar, Hordijk, Sweerts, Van Breemen and Cappenberg1989) studied one of these two pools. As the pools had been strongly affected by acid deposition and enhanced atmospheric S input in the preceding few decades, the results cannot be considered to be representative for natural conditions and local lacustrine sediments under buried conditions. However, the data can be compared with findings for recent sediments within the Rhine-Meuse river system. The acid volatile sulphide content ranged from 22 to 288 μmol/g and the pyrite-S content from 480 to 8000 μg/g. This is lower than the contents found in recent sediments along the large rivers (Table 14). Micromorphologically, pyrite was found as framboids varying in size from 15 to 25 μm associated with organic matter and as tiny single crystals not necessarily associated with organic matter. The slow formation of framboidal pyrite in microniches associated with organic matter particles is assumed to be the pathway of pyrite formation, where pure S is available for the sulphurisation of precursor FeS to pyrite within microniches. The presence of single crystals indicates that some pyrite may also form rapidly. The explanation for the general association of pyrite with organic matter microniches is preferential oxidation of pyrite at locations where pyrite is not protected against O2 intrusion. The organic-bound S constituted 70% of total sedimentary S in the upper 2 cm of the sediment and 50% in the next 8 cm; the organic matter content was about 6% throughout the profile. These data indicate that the occurrence of organic S cannot be neglected.
The speciation of S in these moorland pools is strikingly different from that in Lake Vechten (Table 14; Hordijk et al., Reference Hordijk, Van Engelen, Jonker and Cappenberg1989), which is an isolated sand pit lake and was also subject to enhanced atmospheric S input before being studied. AVS amounted to 60% of total S whereas pyrite-S was less than 10%, organic S was 10–30% and elemental S was negligible at 3%. The conditions for pyrite forming were apparently unfavourable, which Hordijk et al. (Reference Hordijk, Van Engelen, Jonker and Cappenberg1989) attributed to high turnover of organic carbon and/or complete absence of oxygen in these recent sediments.
Calcareous gyttja deposits are found as lacustrine deposits in local depressions left after an ice age, for example as infill of a pingo (Hoek et al., Reference Hoek, Bohncke, Ganssen and Meijer1999) or infillings of oxbow lakes (the latter are part of the Echteld Formation; Smittenberg et al., Reference Smittenberg, Huisman, Veldkamp, Jongmans, Van Os and Huisman1998; Tebbens et al., Reference Tebbens, Veldkamp and Kroonenberg2000). The isotopic composition of these carbonates varied during deposition, depending on the climate and the influx of groundwater. Hoek et al. (Reference Hoek, Bohncke, Ganssen and Meijer1999) measured δ13C values from –8‰ to +1‰ and δ18O values from –7‰ to –3‰ for a continuous gyttja layer of 2 m thickness formed in a local depression (cf. Table 11). The lowest values are associated with colder conditions during the Early Holocene. The isotopic composition of such authigenic carbonates is thus variable and it is difficult to generalise from them. Unfortunately, the abovementioned authors did not study the presence or absence of siderite in addition to that of calcite resulting from the groundwater influx.
Lacustrine sediments are also found in the Amsterdam basin, a glacially excavated basin left after the retreat of the Saalian ice sheet. The Amersfoort basin is another related major basin that accommodated a lake (De Gans et al., Reference De Gans, Beets and Centineo2000). The isotopic composition of carbonate (O, C, Sr) and several other aspects of sediments have been studied in detail in three cores from Amsterdam (Beets & Beets, Reference Beets and Beets2003; Beets et al., Reference Beets, Beets and Cleveringa2006). Three types of lacustrine sediments were found: (1) Late Saalian lake sediments deposited under variable inflow of Rhine water, (2) Late Saalian shallow pool sediments and (3) Eemian lake sediments. The amount and isotopic composition of carbonate differs between these three types (Table 11). The differences in isotopic composition are mainly the result of whether or not there was inflow of river Rhine water, the evaporative concentration in the pools and the climate. The carbonates are authigenic, as evidenced by the Sr content being lower than the marine carbonate content and the strong covariation between δ18O and δ13C and between organic carbon content and δ13C of carbonate. The Eemian lake sediments comprise a layer 1 m thick and contain far more calcareous diatoms and have a higher S content due to some brackish conditions. The presence of authigenic siderite in distinct, somewhat permeable layers in the pool sediments was inferred from the high Fe contents (>2000–3000 ppm) and rapid colouring of the sediment from ochre to brownish-red after exposure to air.
6.3 Aeolian deposits: loess
Loess deposits are composed of loam and stratigraphically classified as the Schimmert Member, being part of the Boxtel Formation. They are found at the surface in southern Limburg and on the southeastern border of the Veluwe. Buried and usually reworked loess deposits are also increasingly being recognised in other parts of the Netherlands (Schokker, Reference Schokker2003). The heavy mineral composition of the loess in southern Limburg is entirely different from that of the underlying formations and the recent deposits of the Meuse river. It is more analogous to the Edelman's (Reference Edelman1933) A group (section 4.1.1). Table 29 gives the standard heavy mineral contents of loess from southern Limburg and the Veluwe area (Mücher, Reference Mücher1973). Mücher (Reference Mücher1973) also noted that the heavy opaque minerals make up around 25% of the total heavy mineral fraction. Vink (Reference Vink1949) pointed out that zircon and rutile are relatively more important as heavy minerals in loess, Brabant loam (as Liempde Member of the Boxtel Formation) and fine cover sands than in coarser sands. The preponderance of zircon and rutile in loess was also observed by Edelman (Reference Edelman1933) and must be attributed to a grain-size effect, as zircon and rutile are usually more fine-grained. According to Druif (Reference Druif1927), the heavy mineral content in the sand fraction of loess varies around 1%.
Table 29. Typical heavy mineral assemblages (in counts of 100) of the aeolian loess deposits (derived from Mucher (1973)).

It is widely recognised that loess deposits have variable carbonate contents, largely due to carbonate leaching, and there is debate about whether decalcification was active prior to the present landscape conditions (Slager et al., Reference Slager, Jongmans, Miedema and Pons1978). Calcite is found in the clay fraction of loess soils, together with small amounts of dolomite (Van der Marel, Reference Van der Marel1950). The ratio of acetic acid soluble carbonate to HCl soluble carbonate is 0.88–0.95 for the clay fraction and around 0.7 for the soil as a whole. This suggests that the percentage of dolomite is systematically lower in the clay fraction than in the coarser fraction. Van Baren (Reference Van Baren1916) studied the relationship between carbonate content and grain size class for deeper loess deposits. He found three layers several metres thick in a quarry: the upper layer was carbonate-free, the second layer contained 6–13% CaCO3 and the third layer an average of 1.5% CaCO3. Most of the CaCO3 was associated with the small particle classes, as the content decreased from 16% for the <10 μm class to 6% for the 100–200 μm class for samples from the second layer. This is broadly similar to the Holocene fluvial sediments of the Rhine (see section 4.4.1). Reinhold (Reference Reinhold1925) and Erdbrink (Reference Erdbrink1953) reported that Ca carbonate can also occur as coatings on quartz grains, which suggests secondary precipitation of carbonate. Slager et al. (Reference Slager, Jongmans, Miedema and Pons1978) also mentioned the occurrence of small amounts of secondary Ca carbonate as channel neocalcitans and nodules. Finally, the occurrence of lössmannetjes (or Lösskindl in German: loess children) is reported in South Limburg and along the Rhine. These are secondary carbonate concretions varying in size from a pea to a human foot (Van Schermbeek, Reference Van Schermbeek1889; Algemeen Handelsblad, Reference Handelsblad1923). Van Baren (Reference Van Baren1916) mentioned that these concretions contain 43–51% Ca carbonate and Mücher (Reference Mücher1973) mentioned that they are particularly found immediately under the boundary between the calcareous and carbonate-free loess.
Reinhold (Reference Reinhold1925) noted that when the carbonate content is left out, the amount of Al2O3 and Fe2O3 in loess is fairly constant, around 15%. This amount includes the silicates and oxyhydroxides. Mineralogical analysis by Druif (Reference Druif1927) revealed that ilmenite and magnetite are prominent Fe oxides. Orthoclase is always present, but microcline and plagioclase vary from not observed to normally present. Muscovite is also frequently observed but biotite is not. The amount of heavy minerals in the sand fraction is also relatively high, 0.4 to 1.3%. The clay fraction is dominated by illite, according to Mücher (Reference Mücher1973). The kandite fraction (kaolinite group) is less than 10% and the smectite fraction is less than 15%. The smectite fraction seems to be somewhat higher in the calcareous loess than in the non-calcareous loess and it increases westward from Sittard. Quartz is also a significant constituent of the clay fraction, with contents 10–15%.
Klein & Griffioen (Reference Klein and Griffioen2008) statistically characterised reworked loess deposits (part of the Boxtel Formation) in the Roer Valley Graben. Both sand and loam samples were classified in this sedimentary facies. Twenty per cent of the loam samples contained between 0.1 and 0.5% pyrite; most of the loam samples and the sand samples contained an amount below the detection limit of 0.1%. This implies that the burial of loess deposits may cause authigenic production of pyrite in a Pleistocene groundwater environment. The carbonate content was around 0.5% for the loam and from below 0.2% to 1% for the sand. Sedimentary organic matter content was generally below 1% for both classes and incidentally up to 5%. It was not indicated whether or not the pyrite was associated with the high sedimentary organic matter content.
A noteworthy phenomenon in the loess of southern Limburg is the presence of a volcanic tuff layer associated with the Eltville Tephra, which is related to Quaternary Eifel volcanism (Meijs et al., Reference Meijs, Mucher, Ouwerkerk, Romein and Stoltenberg1983; Pouclet & Juvigne, Reference Pouclet and Juvigne2009). The layer has been identified as a dark grey layer varying in thickness from millimetres to centimetres in the yellowish loess. Its age is close to 20,000 years and the grain size distribution resembles that of loess. The volcanic deposits are often intermingled with calcareous loess deposits. The layer is composed of pyroclasts and volcanic glass that has altered to an illite-type of clay. The heavy mineral fraction comprises clinopyroxenes (40–60%), olivines (22–50%), brown amphiboles (3–13%), apatite (<9%), some titanite and traces of phlogophite, prehnite and haüyne. Biotite, ∝-tridimite and nepheline are found as light minerals as well as feldspars, which are difficult to separate from loess feldspars. Tephra layers have also been detected in a peat and gyttja-rich infill of a Dutch pingo remnant (Davies et al., Reference Davies, Hoek, Bohncke, Lowe, O'Donnell and Turney2005) and lacustrine deposits in a small lake in the floodplain of the Meuse river (Cremer et al., Reference Cremer, Bunnik, Donders, Hoek, Koolen-Eekhout, Koolmees and Lavooi2010). This tephra material was identified from the occurrence of volcanic glass shards. Such layers may be related to the Laki eruption on Iceland in AD 1783. Further, pumice granules have been observed in fluvial sands of an upper subunit of the Kreftenheije Formation, associated with the 12.9 BP Laacher See volcano eruption (Busschers et al., Reference Busschers, Kasse, Van Balen, Vandenberghe, Cohen, Weerts, Wallinga, Johns, Cleveringa and Bunnik2007).
6.4 Aeolian deposits: dune sand
Holocene dune sand is found along the coast of the North Sea and is geologically classified as the Schoorl Member as well as part of the Zandvoort Member, both of which belong to the Naaldwijk Formation. Two main types of dune sand (and also related beach sand) are distinguished, largely on the basis of their heavy mineral association (Baak, Reference Baak1936; Eisma, Reference Eisma1968): one type occurs north of Bergen and the other type south of Bakkum, with a transitional zone in between. They were described briefly in section 5.1. An identical geographical separation is found for the feldspar, Al and Ca carbonate contents, with Al being proportional to the feldspar content in these sands (Eisma, Reference Eisma1968). The feldspar content in dune sand is 6–8% north of Bergen and 13–17% south of Bakkum and intermediate in between; the feldspar content increases towards the finer sand fraction. In a dune sand sample, Druif (Reference Druif1927) optically observed apatite, muscovite, no biotite and all three feldspars; the latter as expected in decreasing amounts in the sequence orthoclase–microkline–plagioclase.
The dune sands north of Bergen usually contain less than 0.5% CaCO3, with the foredune having a slightly higher content than the dunes farther inland. The carbonate content is high in dune sand south of Egmond and may increase to more than 25% through sorting. This is due to selective erosion of the finer grains and resulting accumulation of coarse shell fragments. For dune slacks (the valleys or troughs between dunes) on the Wadden islands, Sival (Reference Sival1997) found that the CaCO3 generally ranged between 0 and 2% with outliers up to 25% for both topsoil and subsoil samples. This is different from the two dune types described earlier, but dune slacks are also geomorphologically a specific subunit of dunes. Rozema et al. (Reference Rozema, Broekman, Ernst and Appelo1985) investigated decalcification in the coastal dunes of North Holland and Schiermonnikoog based on historical analyses and assuming first-order decalcification with respect to carbonate content. They concluded that decalcification and associated acidification of the topsoil from a pH of 8 to a pH of 4–6 may occur within a time span of several hundreds of years for a layer of 10 cm. Using a similar approach, Stuyfzand (Reference Stuyfzand1993) calculated a rate that is about 10 times faster. Van der Sleen (Reference Van der Sleen1912) previously mentioned that the older, inner coastal dunes have been dolomitised due to rain water leaching based on much lower CaCO3 to MgCO3 ratios at near-similar MgCO3 contents for these sands than for younger, more seaward dune sands.
Enrichment of carbonate may also be observed in the topsoil of dune slacks. Sival et al. (Reference Sival, Mücher and Van Delft1998) studied this phenomenon at three slacks, where all three are former beach plains and nowadays separated from the beach by an embankment or low dunes. Periodic flooding with seawater and aeolian transport were the mechanisms responsible for the enrichment with carbonate shells or fragments and with micrite and silt-sized carbonate particles found in the topsoil. However, authigenic carbonate in the form of nodules, calcitans and crusts was also observed. These were probably formed due to CO2 degassing in association with (1) the capillary rise and evaporation of calcareous groundwater and (2) seepage of carbonate-saturated groundwater and related inundation of the slack.
Stuyfzand et al. (Reference Stuyfzand, Arens and Oost2010) made a comparison on carbonate content and grain size distribution (and other elemental contents) between natural dune sand and man-made sand suppletions at six locations from Wassenaar to Texel. They found that the carbonate content in suppleted dune sand was significantly lower at Wassenaar (where carbonate-rich natural dunes are found) and significantly higher at the other five locations (where carbonate-poor dunes are found). Suppleted sand is also generally coarser, with lower contents of clay and silt. Stuyfzand et al. (Reference Stuyfzand, Arens and Oost2010) also observed higher contents of Ca, Na, K, Fe and Ti in dune sand at Wassenaar than at Bergen and the four more northern locations. This indicates less quartz and more mineralogical richness, probably including feldspars, micas and heavy minerals. Comparably, Van der Sleen (Reference Van der Sleen1912) states that the Fe and Mg contents are highest in between Egmond and Wassenaar, intermediate south of Wassenaar and lowest north of Egmond.
6.5 Aeolian deposits: cover sand
Cover sands are widely found in the Netherlands either at or close to the surface in the Pleistocene part of the Netherlands or buried below Holocene deposits in the Holocene part. Except for the highest areas (ice-pushed ridges), cover sands were deposited under periglacial conditions in a continuous belt north of the loess belt. They are geologically classified as the Wierden Member, which is part of the Boxtel Formation. When lying at the surface, they are subject to pedogenesis, which in recent decades has been enhanced by acid rain. Here, we will focus on the subsoil (>50 cm depth) and buried sediments.
With the exception of the heavy mineral contents, the mineralogy of these cover sands has been poorly studied; more studies have been done on their geochemistry. The lithological composition of the younger cover sands changes from place to place. Crommelin (Reference Crommelin1964) investigated the content and composition of the heavy mineral associations of cover sands in different parts of the Netherlands. Only the 150–210 µm fraction was considered in order to minimise the grain size effects. The differences in the heavy mineral composition of the pre-Weichselian formations in the northern, central and southern parts of the country are reflected in the heavy mineral composition of the superimposed cover sands. Mineralogically, the cover sands may be subdivided into (1) a northern region where garnet, tourmaline and metamorphic minerals are conspicuous, (2) a central region where these three components show lower values but where epidote-saussurite and hornblende-pyroxene groups are more important and (3) a southern region with high percentages of tourmaline and metamorphic minerals.
Van Grinsven et al. (Reference Van Grinsven, Van Riemsdijk, Otjes and Van Breeemen1992) microscopically observed that a series of subsoil samples of acid sandy soils below forest (which are probably cover sands) contained about 10% of predominantly K-feldspar. Nelson & Niggli (Reference Nelson and Niggli1950) isolated opaque grains from a cover sand sample in North Brabant and performed XRD analysis. They found rutile and little ilmenite. Moura & Kroonenberg (Reference Moura and Kroonenberg1990) deduced from the low Rb content of Nuenen aeolian sands (which are nowadays classified under the Boxtel Formation) that contrary to fluvial and marine sands, these sands are virtually free of micas. Mol (Reference Mol2002) geochemically characterised subsoil and topsoil samples below forest in the Pleistocene part of the Netherlands. It is probable that many or all of these samples are cover sands from the Boxtel Formation. Mol distinguished five regions and applied cluster analysis to the dataset. Subsoil samples from eastern North Brabant and the northern parts of the Veluwe and the Utrechtse Heuvelrug lay in one cluster. Those from the southern parts and eastern Gelderland lay in another cluster, whereas those from the northern part of the Pleistocene area of the Netherlands lay in all four clusters. The contents of Na2O, K2O, CaO, MgO, Fe2O3 and TiO2 were generally a factor 1.5–2 higher in the second cluster than in the first cluster, which indicates regional differences in mineralogical richness. In the second cluster, the Na-feldspar content was 5.2 wt% and the K-feldspar content was 6.6 wt%. Schokker et al. (Reference Schokker, Cleveringa, Murray, Wallinga and Westerhoff2005) considered the abundance of Na-feldspars in fine-grained aeolian deposits and related small-scale fluvial and organic deposits as revealed by geochemical analysis of samples from five sediment cores across the Roer Valley Graben. The Na-feldspar content amounted to 9%, coinciding with fine sand or loam.
Klein & Griffioen (Reference Klein and Griffioen2008) characterised a small set of reworked cover sand samples from the Roer Valley Graben. As expected, the pyrite and carbonate contents were mostly below detection limit, but reactive iron ranged up to 0.5% and organic matter was relatively high: 0.5–12% in 40% of the samples. Further, Klein & Griffioen (Reference Klein and Griffioen2012) characterised sand samples from the Boxtel Formation at the Drenthe Plateau and below Holocene deposits in the northern Netherlands. An interesting conclusion is that burial leads to slightly, but statistically significantly, higher pyrite contents, but not to significantly different carbonate contents. Diagenesis during the Holocene resulted in an average pyrite content of 0.2% for the buried samples but the content was below detection limit for samples from the Drenthe Plateau. The latter sand samples from the Boxtel Formation were not further distinguished on their facies, so it is unclear whether all the sand samples were cover sands.
Fluvio-aeolian deposits have often been distinguished by Dutch geologists, for example classified under the former Twente Formation and the recently introduced Boxtel Formation. Schokker et al. (Reference Schokker, Cleveringa, Murray, Wallinga and Westerhoff2005) observed that the Na2O to Al2O3 ratio for sandy fluvio-aeolian sediments from the Boxtel Formation was lower than that of sandy, fluvial sediments from the Sterksel Formation or aeolian sediments from the same Boxtel Formation. This implies lower Na-feldspar contents in favour of other Al minerals and points to different provenances. Hartog et al. (Reference Hartog, Griffioen and Van Bergen2005) characterised a few such shallow samples from the eastern Netherlands on their carbonate isotopes and sedimentary organic matter composition. They found strongly depleted δ18O and δ13C values down to –3‰ and –8‰ associated with anomalously high carbonate contents of about 5%. Such carbonates were concluded to be authigenically produced ferroan ones. This illustrates the importance of local palaeohydrological and diagenetic processes within a fresh groundwater environment.
Locally, red sands are found at the surface in the Veluwe and other locations in the Pleistocene area of the Netherlands, mostly in the cover sands (Weenig, Reference Weenig1951; Bakker & Rogaar, Reference Bakker and Rogaar1993). They occur in the most upstream parts of brook valleys or in dry valleys and extend over several tens to hundreds of square metres. These sands are enriched in goethite, haematite and maghaemite, and are surrounded by similar sands that are not red. The Fe2O3 enrichments above the background level can be as high as 59.7 kg/m2 within 80 cm depth, which is equivalent to about 43,600 mg/kg dry weight. The sands probably originated from groundwater exfiltration under palaeohydrological conditions, supported by the association with gley phenomena (Bakker & Rogaar, Reference Bakker and Rogaar1993).
6.6 Peat
About 9% of the Dutch terrestrial surface consists of peat soils; they are especially present in the western and northern Holocene lowlands but also in the Pleistocene landscape as bogs and as fens in alluvial valleys. The Holocene peat layers are lithostratigraphically classified as the Nieuwkoop Formation and they are found both at the surface and buried. The Eemian Woudenberg Formation is also composed of peat and occurs on the lateral fringe with the marine Eem Formation on top of it. Peat layers are also found at local scale in many other terrestrial formations. Peat is defined in the Netherlands as geomaterial that contains more than 15–30% organic matter, depending on the percentage of clay. This definition implies that the clastic component can be very substantial. Below, we have indicated the kind of peat studied where it must be concluded that Dutch peat has been scarcely studied mineralogically and relationships with type and landscape settings are unclear.
Van Bemmelen (Reference Van Bemmelen1896) visually observed at a peat bog in southeastern Drenthe white, amorphic Fe carbonate as local enrichments in the compacted bottom layer on top of iron ore deposits in a sand layer. The chemical analysis indicated that the white solid is fairly pure FeCO3 with about 5% CaCO3. In another shallower layer he observed vivianite fading into blue that also contained FeCO3. The Fe content of the bottom layer rich in Fe carbonate was higher than that of the vivianite layer and peat in general. Van Bemmelen & Reinders (Reference Van Bemmelen and Reinders1901) also observed whitish crystalline and amorphous Fe carbonate with or without blue-fading vivianite in a peat sublayer under tubular bog iron ore (see below). It is worth pointing out that Smittenberg et al. (Reference Smittenberg, Huisman, Veldkamp, Jongmans, Van Os and Huisman1998) did not observe siderite in addition to calcite and vivianite in Late Pleistocene buried peat layers along the Meuse river whereas they did in the associated gyttja layer (see elsewhere). In this layer, HCO3 is probably present as a result of decomposition of peat, and the supply of Fe may be the limiting factor for siderite formation in the peat. Otherwise, a subtle difference in pH may be limiting. The formation of siderite has been attributed to diagenesis under the influence of exfiltrating Fe-rich groundwater. Van Rossum (Reference Van Rossum1996, Reference Van Rossum1998) found pyrite in basal peat in the western Netherlands using electron microscopy. It occurs as framboids of around 15 μm and octahedral crystals from <1 to 5 μm, where it can be associated with clay minerals or solid organic matter. Similarly, pyrite has also been observed in a peat sample of the Boxtel Formation at the Peel Horst, where it can be as large as 1.2 mm in diameter in association with plant remains (Fig. 15; RGD, 1989).

Fig. 15. SEM backscatter image of a polished thin section showing pyritised plant remains as observed in a buried peat layer of the Boxtel Formation at the Peel Horst (derived from RGD, 1989).
Van Helvoort et al. (Reference Van Helvoort, Griffioen and Hartog2007) analysed a series of Holocene peat samples from the riverine area that were rich in clastic material and originated from peat bogs. They found median values of 2.34% for the pyrite content and 2.39% for the Ca carbonate content. The pyrite content was calculated from the total S content and not corrected for organic S. The weight ratio between organic C and organic S is 60–200:1 for peat in freshwater environments (Price & Casagrande, Reference Price and Casagrande1991; Bartlett et al., Reference Bartlett, Bottrell and Coulson2005), with a typical value of 100:1. For marine peat or freshwater peat that has undergone marine influence, lower ratios are observed: down to 15:1. The organic S content in peat may thus be several tenths of a per cent (or even a few per cent when there has been marine influence), which cannot be neglected when compared to total S measurements for peat. Table 30 summarises statistical data on the reactive solids of peat; the data for the first four groups are more reliable than those for the last two. All these data show that the pyrite content can be high whereas the Ca carbonate content generally remains low. This implies that peat may be susceptible to acidification when pyrite oxidises aerobically and carbonate buffering capacity is insufficient (Van Helvoort et al., Reference Van Helvoort, Griffioen and Hartog2007). Van Bemmelen's (Reference Van Bemmelen1896) observations are thus probably local anomalies. Peat in the regionally occurring Nieuwkoop Formation seems to contain more organic matter than peat that occurs as local layers in the other formations. As presented in Table 25, Vos & De Wolf (Reference Vos and De Wolf1993) found no diatoms in a layer of Phragmite peat from a core in Zeeland. This finding cannot be generalised, as evidenced by Watermann et al. (Reference Watermann, Freund and Gerdes2004) for comparable peat layers in German Holocene coastal deposits: both fresh and saline water types may be present and they probably originate from flood events.
Table 30. Regional characteristics (as percentiles) of the Ca carbonate, clay, organic matter and pyrite contents (in wt%) of peat in different combinations of region and geological unit. The data largely refer to peat deeper than 1 m below surface.

a Probably overestimated because organic S was not taken into account.
b Probably overestimated because exchangeable Ca was not taken into account and gyttja samples were included.
c Probably erroneous because gyttja samples were also classified as peat.
Tambach et al. (Reference Tambach, Veld and Griffioen2009) analysed a sample from a Late Pleistocene buried peat layer on its organic geochemistry. The sample originated from a drilling in the IJssel river valley, Gelderland. They found that it was composed of about 30% pyrolysable carbon and about 70% residual carbon, with the former being dominated by hydrocarbon material instead of CO and CO2. The amount of pyrolysable carbon is calculated from the amount of hydrocarbons, CO and CO2 generated during heating of a sample without any oxidation and the amount of residual carbon is calculated from the amount of CO and CO2 generated upon complete oxidation and heating of the pyrolysis residual fraction (Behar et al., Reference Behar, Beaumont and De B. Penteado2001).
6.7 Bog ore
Strictly speaking, bog ore (or bog iron ore) is not a type of sediment but a diagenetic precipitate or iron containing minerals in a substrate. It was used in the past for iron production, especially in the Vecht area in Overijssel (Joosten, Reference Joosten2004). As its mineralogical and geochemical compositions and other properties are very different than those of regular sediments, it is reviewed separately. When it was exploited as Fe ore, the main constituent was goethite, in fractions varying between 45% and 95% (Joosten, Reference Joosten2004). However, siderite and vivianite can also be major minerals in bog ore (Fig. 16). It has been found in sandy deposits at the bottom of peat layers, in alluvial deposits in brook valleys and also within peat layers as tubular crusts or powder (Van Bemmelen & Reinders, Reference Van Bemmelen and Reinders1901; Reinders, Reference Reinders1902; Booij, Reference Booij1986; Landuydt, Reference Landuydt1990). Its diagenesis has always been linked with exfiltration of Fe-containing groundwater in low-lying areas, where two diagenetic types have been distinguished. It is worth pointing out that bog ore may contain high contents of the trace elements arsenic, cobalt and barium (Van Pruissen & Zuurdeeg, Reference Van Pruissen and Zuurdeeg1988; Joosten, Reference Joosten2004).

Fig. 16. Bog ore with blue-discoloured, oxidised vivianite as found during construction of the entry of the high-speed traintunnel near Leiden, South-Holland.
Van Bemmelen & Reinders (Reference Van Bemmelen and Reinders1901) mentioned that the iron nodules in the sand layer are composed of hard and soft parts, with siderite cementing the sand matrix in the hard parts and Fe oxide being more dominant in the soft parts. They also mentioned the presence of ferro-silicate crystals 18 μm long and 3 μm wide, without further specifying the chemical composition or mineralogy. The diagenesis is due to near-vertical exfiltration of Fe-containing groundwater. This bog ore is found in the upper part of the sand or in the peat immediately above. The tubular crusts in the peat layer were composed of Fe oxide with some Fe phosphate and no Fe carbonate; they were root and stem remnants and had an outer diameter of 4–6 mm and an inner diameter of 0.9–1.5 mm. Van Bemmelen & Reinders (Reference Van Bemmelen and Reinders1901) attributed the existence of tubular Fe oxides to flow of anoxic Fe-rich groundwater from the Pleistocene area through the peat lands, with production of Fe oxides close to the groundwater table under exposure to air. Additionally, Reinders (Reference Reinders1902) explained the existence of powdered bog iron as precipitation of Fe oxides around less woody plants (such as herbs) under similar hydrological conditions. Siderite may be present in a peat sublayer below the bog iron but not in contact with the sand below. These bog ores have always been found near brooks within bog complexes and they are called moerasijzererts in Dutch.
Landuydt (Reference Landuydt1990) characterised the micromorphology of the typical Fe minerals for bog ore along the Nete brooks in the Campine region (Belgium), just south of Breda. Here, goethite usually occurs as coatings on walls and voids or, secondarily, as nodules of <1 mm to 5 cm in diameter, together with ferrihydrite. Siderite is found dispersed as tiny crystals or as spherical, radial nodules 50–200 μm in diameter. Vivianite often occurs as microcrystalline void infillings or aggregates 1–3 mm in diameter.
7 Discussion
The previous sections describe the mineralogy of the different kinds of Cenozoic deposits that are found in the Netherlands together with related recent sediments and suspended matter. This section evaluates the reviewed literature in terms of two major topics: (1) achievements at national level and (2) knowledge gaps that still need to be addressed per group of minerals, as seen from the perspective of the geo-information needs of the Netherlands.
7.1 Mineralogical characterisation of Dutch sediments and suspended matter
Many of the studies reviewed dealt with small amounts of samples: from a few to several dozens of samples. Notable exceptions are the studies dealing with long borehole cores, and the geotop programme of the TNO Geological Survey (Griffioen et al., Reference Griffioen, Klein and Van Gaans2012 and related reports). For the latter, a systematic geochemical characterisation is currently in progress for the first tens of metres of the subsurface as part of a broad geoscientific characterisation programme (Van der Meulen et al., Reference Van der Meulen, Doornenbal, Gunnink, Stafleu, Schokker, Vernes, Van Geer, Van Gessel, Van Heteren, Van Leeuwen, Bakker, Bogaard, Busschers, Griffioen, Gruijters, Kiden, Schroot, Simmelink, Van Berkel, Van der Krogt, Westerhoff and Van Daalen2013). Under this programme, specific geochemical and grain size analyses are being recalculated to obtain contents of Fe sulphide, carbonates, organic matter, non-sulphide reactive Fe minerals and clay (Van Gaans et al., Reference Van Gaans, Griffioen, Mol and Klaver2011). Little insight thus exists on the spatial representativeness of the individual studies for other geographical areas. This hampers environmental management of the Netherlands at the local to national level, as the mineralogical information is useful for the reasons mentioned in the introduction.
The complete suite of manuscripts reviewed also indicates that until the 1970s the mineralogical characterisation entailed using optical microscopes. Since then, geochemical techniques have been applied much more frequently, although wet chemical extraction techniques had also been used previously. The most densely studied areas are the Scheldt systems. This seems to be due to a combination of reasons: (1) research groups from both the Netherlands and Belgium have been active here, (2) the Western Scheldt system was severely polluted with nutrient and trace metals and is still recovering from this, and (3) there is academic interest in biogeochemical cycling in these estuarine environments, particularly of carbon and sulphur. The paucity of studies on glacial sediments and peat is striking, given the widespread occurrence of the former and the importance of the latter as soil and landscape types in the Netherlands. Hardly any mineralogical studies have been done in the provinces of North Holland, Flevopolder, Friesland and Groningen (outside the Wadden Sea) and coastal dunes. Assumptions on the mineralogy of the sediments present in these provinces must thus be based on spatial extrapolation from other parts of the Netherlands, together with more general mineralogical insights. Finally, the number of studies that deal with sediments deeper than 30 m is also limited.
7.2 Heavy minerals
Heavy mineral associations have been characterised routinely in the past for many kinds of sediments in many kinds of borehole cores. Regrettably, the absolute amounts of these minerals were rarely measured. Such data would be useful to better estimate the role of weathering of these minerals relative to that of micas and plagioclase in relation to acid buffering in the acid sandy soils that are frequently found in the Netherlands (Mol, Reference Mol2002; Van der Salm & Verstraten, Reference Van der Salm and Verstraten1994) and evolution of the groundwater composition in non-calcareous aquifers (Griffioen et al., Reference Griffioen, Vermooten and Janssen2013). The systematic removal of individual heavy minerals during weathering is a topic that deserves further attention because errors can be made in the stratigraphical interpretation when this phenomenon is overlooked.
Most previous work on heavy minerals in the Rhine River (e.g. Van Andel, Reference Van Andel1950; Westerhoff et al., Reference Westerhoff, Kemna and Boenigk2008; Krippner & Bahlburg, Reference Krippner and Bahlburg2013) focused on transparent minerals (e.g. garnet, epidote, hornblende, tourmaline and zircon), their proportions and/or U–Pb age dating of zircons.The latter method is valuable for characterisering the provenance areas of sediments. It brought Krippner & Bahlburg (2013) to question the commonly made assumption of a direct delivery of detritus to the downstream Rhine river system from the Alps during at least some parts of the Pleistocene. This would also necessitate the re-evaluation of explanations on the provenance of Dutch sediments.
7.3 Clay minerals
Several studies have used XRD to examine the clay mineralogy of fluvial and marine sediments, but the glacial sediments are under-represented. The outcomes indicate that a mixture of clay minerals is always present, with variations in the individual fractions. Systematic differences have been noted between fluvial clays, alluvial clays and marine Tertiary or Quaternary clays. Typically, from 5 to 20 samples were characterised in the individual studies. One can but wonder how much practical geo-information insight would have been obtained if much larger numbers of samples had been analysed. Although considerable advances have been made in the level of detail of XRD analysis of clay minerals, many more insights into the geological controls of sedimentary clay mineralogy could be obtained by increasing the density of sampling of the sedimentary records (Zeelmaekers, Reference Zeelmaekers2011).
7.4 Feldspars and micas
The presence of feldspars and micas in marine sediments has hardly been studied, even though it is relevant within the context of long-term weathering of these sediments, as feldspars may be a potential source of major elements such as Na and K, and trace elements such as Ba. It would also improve understanding of diagenesis in groundwater environments as an intermittent stage in the diagenetic evolution from the original sedimentary environment to the conditions present in deep oil and gas reservoirs. Another shortcoming in the mineralogical characterisation is the lack of insight into the occurrence of black biotite in addition to silverish micas. A few studies have pointed out the presence of biotite, but these characterised only small numbers of samples. Biotite is more susceptible to weathering than muscovite, however, and it may be a source of Mg, Fe and also F in pore water.
7.5 Ca carbonates
The review indicates that the origin of Ca carbonates may be diverse within a sediment type: primary rock carbonates and secondary soil carbonates from the hinterland are transported by the rivers, together with possibly authigenic Ca carbonate. These are mixed with other carbonates in estuaries and just offshore. Calcium carbonate in the marine environment also refers to old rock carbonates (but which may be from another provenance than the carbonates transported in the rivers), authigenic young shells and their fragments and in situ precipitated Ca carbonate (where dissolution may also take place, particularly in supratidal flats). The different kinds of Ca carbonate present may have different ranges in δ13C-CO3, δ18O-CO3 and 14C age. As a result, many sediments will be an isotopic mixture of carbonates. The presence of different kinds of carbonates within an individual sediment that may be involved in dissolution processes has implications for groundwater dating, for example on the need to correct from apparent age to true age when using 14C analysis.
Another complicating issue for Ca carbonates is the presence of dolomite versus calcite and aragonite. It is clear that dolomite plays a role in Dutch marine and fluvial sediments. It seems likely that calcite and aragonite are more susceptible to leaching than dolomite because of the differences in kinetic dissolution rates. A sediment would thus gradually become more enriched in dolomite than in pure Ca carbonates. Ritsema & Groenenberg (Reference Ritsema and Groenenberg1993) observed this phenomenon in a marine clay soil and Van der Sleen (Reference Van der Sleen1912) described it for the older, inner coastal dunes at a few locations along the western coast, but it has not yet been generally acknowledged and has been studied even less.
7.6 Reactive Fe minerals
The occurrence of reactive Fe minerals has been studied frequently but enigmatic questions still remain for the Dutch settings. The focus has been on Fe sulphides in the Eastern and Western Scheldt estuaries, but studies have also examined Fe sulphides in other environments. There are clear indications that pyrite is present not only in marine sediments or terrestrial sediments that have been subject to marine environments, but also in fluvial environments that remained terrestrial for more than 1 million years (e.g. the Waalre and Sterksel Formations in the southern Netherlands). A source for sulphide must have been present to diagenetically produce such pyrites: we believe that it is SO4 dissolved in the river Rhine (or other rivers), as the SO4 concentration in the Rhine under natural conditions was around 35 mg/L (Griffioen et al., Reference Griffioen, Passier and Klein2008). Given sufficient time and sufficient river bank infiltration, the Rhine may yield sufficient SO4 to produce regionally dispersed pyrite in aquifer sediments.
Less well studied in the Netherlands are siderite, Fe oxyhydroxides, glauconite and vivianite. These minerals can be grouped together as non-pyrite reactive iron, which is being statistically characterised at regional scale under the ongoing national geotop programme (Van Gaans et al., Reference Van Gaans, Griffioen, Mol and Klaver2011; Van der Meulen et al., Reference Van der Meulen, Doornenbal, Gunnink, Stafleu, Schokker, Vernes, Van Geer, Van Gessel, Van Heteren, Van Leeuwen, Bakker, Bogaard, Busschers, Griffioen, Gruijters, Kiden, Schroot, Simmelink, Van Berkel, Van der Krogt, Westerhoff and Van Daalen2013). Based on the existing data on Dutch sediments, it is impossible to make any further distinctions between these minerals even though their geochemical properties are very different. Additional insights would therefore be very welcome for these sediments on this type of minerals.
Siderite has been incidentally identified when focusing on concretions. The environment in which such concretions form will be somewhat exceptional and the insights obtained into the siderite diagenesis cannot be extrapolated to the geoscientific settings of similar sediments in which these concretions do not occur. Insight into the diagenesis of dispersed siderite is needed, amongst other reasons to better understand its importance in controlling the groundwater chemistry, as much groundwater in the Netherlands is saturated or supersaturated in siderite but not necessarily saturated in calcite (Griffioen et al., Reference Griffioen, Vermooten and Janssen2013).
Iron oxyhydroxides are important with respect to the buffering capacity of sediments and soils against reduction, and as sorbent for trace elements and other solutes. There is no general insight into the presence and related diagenesis of these minerals in Dutch sediments deeper than the soil horizons (Fig. 17). The presence of detrital Fe oxides has been shown microscopically for Meuse sediments in southern Limburg and a few more sites, but the spatial representativeness of these observations is largely limited to the southeastern part of the Netherlands. Insight into the systematic presence or absence of detrital Fe oxyhydroxides in Dutch sediments is crucial, but is currently lacking. Extractions have been used to indicate the amount of Fe oxyhydroxides in Dutch sedimentary environments, but there is always a possibility of interference from other solids during the Fe oxide extraction (Martin et al., Reference Martin, Nirel and Thomas1987; La Force & Fendorf, Reference La Force and Fendorf2000). The results of these extractions also suggested the presence of Fe oxyhydroxides under SO4-reducing conditions (Canavan et al., Reference Canavan, Slomp, Jourabchi, Van Cappellen, Laverman and Van den Berg2006). This is an important observation because it shows that not all Fe oxyhyroxides present can be microbially reduced for the given time scale and that under reduced conditions an oxic mineral remains present that will be part of the sediment sorption capacity. The presence of Fe oxyhydroxides under SO4-reducing conditions needs to be confirmed using other characterisation techniques.

Fig. 17. Local, horizontal enrichments of Fe-oxyhydroxides as well as Mn-oxyhydroxides in sand of the Sterksel Formation as found in the Maalbeek quarry (Limburg). It is yet unknown how and when these oxyhydroxides were formed diagenetically.
7.7 Peat and sedimentary organic matter
The characterisation of peat and sedimentary organic matter in Dutch conditions is poor. A very limited series of studies applied mineralogical or geochemical characterisation methods to peat. The early studies (around 1900) on vivianite and siderite in peat are still unique. It must be remembered that most peats are not completely composed of organic matter but may contain more than 50% of clastic material as well. Knowledge about the mineralogical composition of this clastic component is important as this composition affects the reactivity of the peat in different ways. The findings may help to address technical issues such as subsidence of peatlands, the natural background load of nutrients in the associated surface water system, nature restoration, etc.
Only a few studies have addressed the composition of organic matter; most have focused on fresh sediments and suspended matter in the southern North Sea. Studies of the type performed for the Dutch marine environment would also be very useful for the Dutch limnological environment, for which there are still surface water quality issues (Van Puijenbroek et al., Reference Van Puijenbroek, Jnse and Knoop2004; Janse et al., Reference Janse, Scheffer, Lijklema, Van Liere, Sloot and Mooij2010). Further, microcosm respiration experiments on small numbers of samples have yielded information on the redox reactivity of different kinds of sediments present. These experiments have elucidated the role of organic matter oxidation versus oxidation of reactive Fe(II) minerals, which is amongst other things relevant for aquifer storage and recovery (Prommer & Stuyfzand, Reference Prommer and Stuyfzand2005; Antoniou et al., Reference Antoniou, Van Breukelen, Putters and Stuyfzand2012). More generally, insight into the functional and molecular composition of sedimentary organic matter would be fruitful to clarify the reactivity and diagenetic history of sediments, in particular of Dutch sediments.
Acknowledgments
The librarians of Deltares are gratefully acknowledged for collecting many old and very old references as well as some more recent ones. We thank our colleagues Aleid Bosch, Wim Dubelaar, Sytse van Heteren (all TNO) and Harry Veld (Deltares) for commenting on an earlier version of the manuscript or parts of it. Holger Cremer (TNO) and Albert Oost (Deltares) are thanked for introducing us to the world of Dutch diatoms and shells, respectively. The two reviewers and editor Ronald van Balen are thanked for their very precise and constructive comments. Joy Burrough was the language editor of a near-final draft of the manuscript. Reference is made to the website www.dinoloket.nl as it was in August 2014.