No CrossRef data available.
Article contents
Uncoiling of DNA by Double Chained Cationic Surfactants
Published online by Cambridge University Press: 10 February 2011
Abstract
Distearyldimethylammonium (DSDMA) X (X− = OH, H2PO4) interact with double stranded T4 DNA (166 kilobase pairs) below and above the CMC (1.5×10−6). Below the CMC of either DSDMA X where the cationic double-chained surfactants are in the monomeric state, T4 DNA and DSDMA+form a compact complex where all surfactant molecules are bound. Close to the CMC, particularly for DSDMA OH but also in the presence of 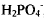 , T4 DNA exhibits a condensed (globule) conformation, though some DNA molecules are in the more extended DNA conformation. Above the CMC of DSDMA X a plateau is reached (up to 3.0 × 10−6 M surfactant) revealing a penetration of DSDMA molecules into the DNA globules resulting in a loosening of the tightened state of DNA. The various stages of the transition from the condensed coil to the extended state of T4 DNA with changing DSDMA X concentrations was monitored by static and inelastic light scattering experiments which were supplemented by small-angle X-ray scattering measurements.
, T4 DNA exhibits a condensed (globule) conformation, though some DNA molecules are in the more extended DNA conformation. Above the CMC of DSDMA X a plateau is reached (up to 3.0 × 10−6 M surfactant) revealing a penetration of DSDMA molecules into the DNA globules resulting in a loosening of the tightened state of DNA. The various stages of the transition from the condensed coil to the extended state of T4 DNA with changing DSDMA X concentrations was monitored by static and inelastic light scattering experiments which were supplemented by small-angle X-ray scattering measurements.
- Type
- Research Article
- Information
- Copyright
- Copyright © Materials Research Society 1997


