Published online by Cambridge University Press: 10 February 2011
We have investigated the interaction of the actinyl ion, 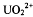 , with silica, alumina, and montmorillonite surfaces under ambient atmosphere and aqueous conditions using x-ray Absorption Fine Structure (XAFS) Spectroscopy. In acid solution (pH ∼ 3.5), the uranyl ion shows a strong interaction with the silica and alumina surfaces, and a relatively weak association with the montmorillonite surface. The extent of direct surface interaction is determined by comparing structural distortions in the equatorial bonding environment of the uranyl ion relative to the structure of a “free” uranyl aquo complex. Based on this formalism, surface complexation on silica and alumina occurs through an inner-sphere mechanism with surface oxygen atoms binding directly to the equatorial region of the uranyl ion. In contrast, sorption on montmorillonite occurs by an outer sphere mechanism in which the uranyl ion retains the simple aquo complex structure and binds to the surface via ion-exchange. In near-neutral solutions (pH ∼ 6), sorption on all of the materials is dominated by an inner-sphere mechanism. The formation of surface oligomeric species is also observed on silica and alumina.
, with silica, alumina, and montmorillonite surfaces under ambient atmosphere and aqueous conditions using x-ray Absorption Fine Structure (XAFS) Spectroscopy. In acid solution (pH ∼ 3.5), the uranyl ion shows a strong interaction with the silica and alumina surfaces, and a relatively weak association with the montmorillonite surface. The extent of direct surface interaction is determined by comparing structural distortions in the equatorial bonding environment of the uranyl ion relative to the structure of a “free” uranyl aquo complex. Based on this formalism, surface complexation on silica and alumina occurs through an inner-sphere mechanism with surface oxygen atoms binding directly to the equatorial region of the uranyl ion. In contrast, sorption on montmorillonite occurs by an outer sphere mechanism in which the uranyl ion retains the simple aquo complex structure and binds to the surface via ion-exchange. In near-neutral solutions (pH ∼ 6), sorption on all of the materials is dominated by an inner-sphere mechanism. The formation of surface oligomeric species is also observed on silica and alumina.