Published online by Cambridge University Press: 21 February 2011
Eh-pH diagrams are useful for comparing the probable geochemical behavior of many elements including U and the transuranics Np, Pu, Am. Revised Eh-pH diagrams have been constructed for U, Np, Pu and Am at 25 C, 1 bar conditions. These diagrams, based on new and revised thermodynamic data, include aqueous and solid species in the generic system M-C-O-H. The species M(OH)− is considered herein, although it's existence has not been verified.
Aqueous U(VI) species are dominated by 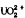 below pH 5 and by carbonate complexes at higher pH. U(IV) species include important fields of UO2,
below pH 5 and by carbonate complexes at higher pH. U(IV) species include important fields of UO2, 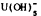 and U(OH)4. If Si is present, USiO4 may be important. U3O8, occurs between the U(IV) and U(VI) species.
and U(OH)4. If Si is present, USiO4 may be important. U3O8, occurs between the U(IV) and U(VI) species.
Np(IV) solid species are problematic. NpO2 covers a wide range of Eh-pH, but its precursors, NpO. (OH)2 and Np(OH)4 cover more restrictive Eh-pH ranges with 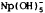 more important. This increases potential for radionuclide migration. Np(V, VI) carbonate complexes are important only at high Eh.
more important. This increases potential for radionuclide migration. Np(V, VI) carbonate complexes are important only at high Eh.
Pu(IV) species are dominated by PuO2, but Pu(OH)4 does not occupy as much Eh-pH space, being replaced in large part by 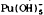 . With carbonate present, Pu(III) species also replace part of the Pu(OH)4 field, and Pu2 (CO3)3 becomes important. Pu(VI) carbonate complexess occur only at high Eh.
. With carbonate present, Pu(III) species also replace part of the Pu(OH)4 field, and Pu2 (CO3)3 becomes important. Pu(VI) carbonate complexess occur only at high Eh.
Am(III) species dominate Eh-pH space in the system Am-C-O-H, with Am2 (CO3)3 a major phase. Under carbonate-poor conditions, Am(OH) 3 is important. AmO2 occurs at high Eh, pH, and 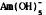 at high Eh and very high pH.
at high Eh and very high pH.
The importance of the M(OH)4 precursors to MO2 species is the enhanced possibility of radionuclide migration from buried spent fuel rods, even under reducing condtions.