Published online by Cambridge University Press: 15 February 2011
Chemical reference tables state that the standard potential for the reaction of Pt with water, Pt + 2H2O → Pt(OH)2 + 2H+ + 2e−, is 0.98 V, and electrochemical studies propose that this reaction may occur at potentials as low as 0.8 V [1–5]. Using dispersive x-ray absorption finestructure (XAFS) spectroscopy [6], we have directly probed the structural evolution of a Pt catalyst operating in-situ in a polymer electrolyte [7] fuel cell during cyclic voltammetry. The changes in the number of Pt and O nearest-neighbors and the Pt charge demonstrate a close correspondence with features in the voltammogram. Because dispersive XAFS is very sensitive to detecting structural changes, we have been able to detect the presence of chemisorbed oxygen at potentials of 0.6–0.9 V in the anodic sweep. Since double-layer charging is regarded as the only process in this region for bulk Pt [3–5], these results may reflect a limitation of previous (indirect) studies on Pt electrochemistry, or they may indicate that these clusters are different from their bulk metal counterparts. Exploiting the timeresolving capability of dispersive XAFS, we also monitored changes in the Pt charge and the number of O and Pt nearest-neighbors during the electrochemical oxidation and reduction of the Pt clusters in real-time. The results are inconsistent with those expected from the placeexchange mechanism [8–11] for the formation of the surface oxide on bulk Pt electrodes in aqueous solution;
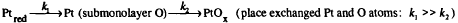
Our current model for understanding these behaviors is that, relative to bulk Pt, unusual types of surface sites play a major role in determining the reactivity of these clusters.