No CrossRef data available.
Published online by Cambridge University Press: 15 February 2011
Experimental data for the exchange of protons between proteins and the aqueous solvent is re-examined using a fractal model. The fraction of protons unexchanged from the protein, f is seen to follow a stretched exponential, 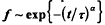 at long times. For the protein, lysozyme, data over a range of temperatures were considered and accurate fits were obtained with a single, unadjusted scaling exponent, α. The time constant, τ, followed an Arrhenius law and gave an activation energy comparable to that obtained for free peptide exchange. A model is proposed where proton exchange occurs as a result of solvent reacting at the fractal surface of the protein. The protein itself is not treated as static but has units that diffuse to the surface. The diffusion of these units in the long time domain is assumed to be classical. In this model the scaling exponent, α, is related to the spectral dimension of the system. Treating the problem as a reaction of the type A+B→C in a confined region, the exponent is given by: α = (3 − ds)/4 where dS/4 is the fractal surface dimension of the protein. Using the value of 2.17 previously established for the surface dimension of lysozyme, data at 6 different temperatures could be fit with the corresponding α of 0.21. These results show that the fractal structure of a protein can influence difflusional processes of small molecules associating with the protein.
at long times. For the protein, lysozyme, data over a range of temperatures were considered and accurate fits were obtained with a single, unadjusted scaling exponent, α. The time constant, τ, followed an Arrhenius law and gave an activation energy comparable to that obtained for free peptide exchange. A model is proposed where proton exchange occurs as a result of solvent reacting at the fractal surface of the protein. The protein itself is not treated as static but has units that diffuse to the surface. The diffusion of these units in the long time domain is assumed to be classical. In this model the scaling exponent, α, is related to the spectral dimension of the system. Treating the problem as a reaction of the type A+B→C in a confined region, the exponent is given by: α = (3 − ds)/4 where dS/4 is the fractal surface dimension of the protein. Using the value of 2.17 previously established for the surface dimension of lysozyme, data at 6 different temperatures could be fit with the corresponding α of 0.21. These results show that the fractal structure of a protein can influence difflusional processes of small molecules associating with the protein.