Published online by Cambridge University Press: 25 February 2011
In order to develop new precursors for the preparation of titanium nitride, a Ti alkoxide-nitrogen containing polyol condensation product was prepared and heat-treated. A stoichiometric mixture of titanium tetra-isopropoxide (Ti(OC3H7)4) and triethanolamine (N(CHM2H4OH)3) was heated at ca. 200°C under reduced pressure. The distilled liquid was isopropyl alcohol and its amount indicated that the transesterification reaction proceeded almost quantitatively.
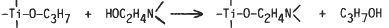
The condensation product was a transparent glassy solid on cooling to room temperature. Thermal treatment of the condensation product at 1600°C in a nitrogen flow yielded titanium nitride (TiN1-x-yCxOy) as the main phase with a small amount of Ti3O5. The thermal treatment for a longer time reduced the amount of the oxide. The heat treatment of the product in an Ar flow also yielded nitrogen containing ceramics, which indicated the effectiveness of nitrogen in the condensation product during the conversion.