No CrossRef data available.
Published online by Cambridge University Press: 22 February 2011
The instantaneous impedance of AL in 1N NaCl at various polarization tines has been measured using LapLace transformation method. In an instant of beginning polarization, the dependance of interfacial resistance, Rf, on polarization current, ip, follows:
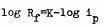
Interfacial capacitance, Cf, however, indepenrs of ip, only depends on film's thickness. In the course of polarization, Rf changed hardly with time, Cfchanged greatly. This is due to little difference between the resistance on corroded region and the resistance on the region with film, and great difference capacitance on both regions. A reasonable model has beon pronosed to explain the experimental results.