No CrossRef data available.
Article contents
Arrested Solid-Solid Phase Transition in Semiconductor Nanocrystals
Published online by Cambridge University Press: 28 February 2011
Abstract
CdS crystallites of 40 and 45 Å average diameter have been studied under hydrostatic high pressure by optical absorption. The optical absorption onset is observed to shift to higher energy with pressure according to
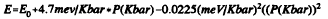
This is exactly the same shift with pressure as is observed in the bulk. The solid-solid phase transition from the zincblende to the NaCl phase is observed as an abrupt change in the intensity, intercept, and functional form of the absorption onset. The phase transition, which occurs at 27Kbar in bulk CdS, is shifted to 85 Kbar in nanocrystals stabilized with polyphosphate, and to 60 Kbar in nanocrystals surface derivatized with EDTA. It thus appears possible to control the relative stability of the crystalline phases of a nanocrystal by chemical manipulation of the surface.
- Type
- Research Article
- Information
- Copyright
- Copyright © Materials Research Society 1991


