No CrossRef data available.
Article contents
An Antimony Planar Diffusion Source
Published online by Cambridge University Press: 25 February 2011
Abstract
Antimony is a useful n-type dopant for buried layers, due to its low rate of lateral diffusion in subsequent processing steps. To date, no solid planar diffusion source has been available. Reported here is the development of such a source.
The source is made by high temperature synthesis in a hot press. A mixture of antimony trioxide and silicon powders is placed in the press, and heated under pressure. The reaction
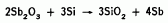
results in a solidified product. Wafers are sliced from this product to form the actual planar diffusion sources.
The sources are used in a two-step doping process: a predeposition followed by a drive-in. Oxygen is required in both steps, due to the chemistry involved. Typical properties of the doped silicon are: sheet resistance, 10 Ω/square, junction depth, 4–6 microns, and antimony surface concentration. 3–8 × 1019/cm3.
- Type
- Research Article
- Information
- Copyright
- Copyright © Materials Research Society 1989


