Crossref Citations
This article has been cited by the following publications. This list is generated based on data provided by
Crossref.
Wieland, E.
Macé, N.
Dähn, R.
Kunz, D.
and
Tits, J.
2010.
Macro- and micro-scale studies on U(VI) immobilization in hardened cement paste.
Journal of Radioanalytical and Nuclear Chemistry,
Vol. 286,
Issue. 3,
p.
793.
Tits, Jan
Geipel, Gerhard
Macé, Nathalie
Eilzer, Manuela
and
Wieland, Erich
2011.
Determination of uranium(VI) sorbed species in calcium silicate hydrate phases: A laser-induced luminescence spectroscopy and batch sorption study.
Journal of Colloid and Interface Science,
Vol. 359,
Issue. 1,
p.
248.
Cantrell, Kirk J.
Deutsch, William J.
and
Lindberg, Mike J.
2011.
Thermodynamic Model for Uranium Release from Hanford Site Tank Residual Waste.
Environmental Science & Technology,
Vol. 45,
Issue. 4,
p.
1473.
Gaona, Xavier
Kulik, Dmitrii A.
Macé, Nathalie
and
Wieland, Erich
2012.
Aqueous–solid solution thermodynamic model of U(VI) uptake in C–S–H phases.
Applied Geochemistry,
Vol. 27,
Issue. 1,
p.
81.
Tits, J.
Gaona, X.
Macé, N.
Kulik, D.
Stumpf, T.
Walther, C.
Geipel, G.
and
Wieland, E.
2012.
Proceedings of the 10th International Congress for Applied Mineralogy (ICAM).
p.
699.
Wieland, E.
Dähn, R.
Gaona, X.
Macé, N.
and
Tits, J.
2013.
Cement-Based Materials for Nuclear Waste Storage.
p.
93.
Macé, Nathalie
Wieland, Erich
Dähn, Rainer
Tits, Jan
and
Scheinost, Andreas C.
2013.
EXAFS investigation on U(VI) immobilization in hardened cement paste: influence of experimental conditions on speciation.
Radiochimica Acta,
Vol. 101,
Issue. 6,
p.
379.
Cachoir, C.
Mennecart, Th.
and
Lemmens, K.
2014.
Effect of cement water on UO2 solubility.
MRS Proceedings,
Vol. 1665,
Issue. ,
p.
267.
Mani, D.
and
Kumar, Chitranjan
2014.
Biotechnological advances in bioremediation of heavy metals contaminated ecosystems: an overview with special reference to phytoremediation.
International Journal of Environmental Science and Technology,
Vol. 11,
Issue. 3,
p.
843.
Mennecart, Thierry
Cachoir, Christelle
and
Lemmens, Karel
2015.
Influence of the alpha radiation on the UO2 dissolution in high pH cementitious waters.
Journal of Radioanalytical and Nuclear Chemistry,
Vol. 304,
Issue. 1,
p.
61.
Cachoir, Christelle
Salah, Sonia
Mennecart, Thierry
and
Lemmens, Karel
2016.
Uranium Dissolution in Hyperalkaline TMA-OH Solutions: Preliminary Results.
Procedia Chemistry,
Vol. 21,
Issue. ,
p.
306.
Ochs, Michael
Vriens, Bas
and
Tachi, Yukio
2018.
Retention of uranium in cement systems: effects of cement degradation and complexing ligands.
Progress in Nuclear Science and Technology,
Vol. 5,
Issue. 0,
p.
208.
Ma, Bin
Charlet, Laurent
Fernandez-Martinez, Alejandro
Kang, Mingliang
and
Madé, Benoît
2019.
A review of the retention mechanisms of redox-sensitive radionuclides in multi-barrier systems.
Applied Geochemistry,
Vol. 100,
Issue. ,
p.
414.
Philipp, Thimo
Shams Aldin Azzam, Salim
Rossberg, André
Huittinen, Nina
Schmeide, Katja
and
Stumpf, Thorsten
2019.
U(VI) sorption on Ca-bentonite at (hyper)alkaline conditions – Spectroscopic investigations of retention mechanisms.
Science of The Total Environment,
Vol. 676,
Issue. ,
p.
469.
Mancini, A.
Wieland, E.
Geng, G.
Lothenbach, B.
Wehrli, B.
and
Dähn, R.
2021.
Fe(II) interaction with cement phases: Method development, wet chemical studies and X-ray absorption spectroscopy.
Journal of Colloid and Interface Science,
Vol. 588,
Issue. ,
p.
692.
Phillip, E.
Khoo, K.S.
Yusof, M.A.W.
and
Abdel Rahman, R.O.
2022.
Mechanistic insights into the dynamics of radionuclides retention in evolved POFA-OPC and OPC barriers in radioactive waste disposal.
Chemical Engineering Journal,
Vol. 437,
Issue. ,
p.
135423.
Baur, Sandra
Brix, Kristina
Feuerstein, Aylin
Janka, Oliver
and
Kautenburger, Ralf
2022.
Retention of waste cocktail elements onto characterised calcium silicate hydrate (C–S–H) phases: A kinetic study under highly saline and hyperalkaline conditions.
Applied Geochemistry,
Vol. 143,
Issue. ,
p.
105319.
Stietz, Janina
Amayri, Samer
Häußler, Verena
Scholze, Raphael
and
Reich, Tobias
2023.
The Uptake of Actinides by Hardened Cement Paste in High-Salinity Pore Water.
Minerals,
Vol. 13,
Issue. 11,
p.
1380.
Brix, Kristina
Haben, Aaron
and
Kautenburger, Ralf
2023.
Time-Dependent Retention of a Mixture of Cs(I), Sm(III), Eu(III) and U(VI) as Waste Cocktail by Calcium Silicate Hydrate (C-S-H) Phases.
Minerals,
Vol. 13,
Issue. 12,
p.
1469.
Macé, Nathalie
Page, Jacques
and
Reiller, Pascal E.
2023.
Uranium(VI) Sorption onto Hardened Cement Paste under High Saline and Alkaline Conditions.
Minerals,
Vol. 13,
Issue. 3,
p.
325.
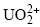 could control U(VI) uptake by C-S-H phases.
could control U(VI) uptake by C-S-H phases.