No CrossRef data available.
Published online by Cambridge University Press: 28 February 2011
The upper shelf fracture toughness of ultra high strength steels is dependent on both the microstructure, which is determined by composition and heat treatment, and on the inclusions present in the steel. The inclusions In ultra high strength steels are typically oxides and sulfides [1]. In most ultra high strength steels the sulfides are manganese sulfides, although depending on the composition of the steel and the melt practice used, other sulfides are found, such as chromium sulfide, calcium sulfide and lanthanum oxy-sulfide [2]. If the inclusions can be regarded as pre-existing voids then the inclusion volume fraction and spacing appear to be sufficient to characterize the inclusion population from the standpoint of fracture toughness [3,4]. The purpose of this paper is to discuss results which show sulfur can be gettered as particles which are much more resistant to void nucleation than manganese sulfides and that this increased resistance to void nucleation can result in vastly improved upper shelf fracture toughness. In particular, when HY180 steel contains manganese sulfides the fracture toughness is about 250 MPa 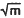 but when the sulfur is gettered as particles containing titanium, carbon and sulfur the fracture toughness of HY180 steel will approach 550 MPa
but when the sulfur is gettered as particles containing titanium, carbon and sulfur the fracture toughness of HY180 steel will approach 550 MPa 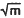 . These particles, believed to be titanium carbosulfides, are much more resistant to void nucleation than manganese sulfides and this increased resistance to void nucleation appears to be the reason for the improved fracture toughness.
. These particles, believed to be titanium carbosulfides, are much more resistant to void nucleation than manganese sulfides and this increased resistance to void nucleation appears to be the reason for the improved fracture toughness.