Published online by Cambridge University Press: 10 August 2011
Protons can fit into the perovskite structure because the O-O distance is approximately that of ice. However, to achieve charge balance, -2e charge must be added for every two protons added. If O2- vacancies are introduced, for instance by doping barium cerate with yttrium to obtain 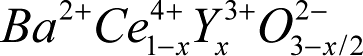 (BCY), each filled vacancy will add -2e charge. Proton concentration and conductivity can be increased by exposing BCY to steam molecules, which dissociate into 2 protons and an O2- ion. Our impedance spectroscopy measurements and solid oxide fuel cell (SOFC) work shows that exposure to H2 on the anode side can add protons. This requires addition of O2- ions on the cathode side from O2 in air. Alternatively, electrons could be added because BCY is a weak electronic semiconductor. BCY is a hole conductor, and this conductivity decreases upon exposure to steam or H2. From our analysis of V(i) curves for SOFCs with BCY electrolyte, at low H2 concentration, increasing this concentration decreases electronic conductivity somewhat, consistent with adding electrons and reducing hole carrier concentration. At higher H2 concentrations, electronic conductivity remains constant.
(BCY), each filled vacancy will add -2e charge. Proton concentration and conductivity can be increased by exposing BCY to steam molecules, which dissociate into 2 protons and an O2- ion. Our impedance spectroscopy measurements and solid oxide fuel cell (SOFC) work shows that exposure to H2 on the anode side can add protons. This requires addition of O2- ions on the cathode side from O2 in air. Alternatively, electrons could be added because BCY is a weak electronic semiconductor. BCY is a hole conductor, and this conductivity decreases upon exposure to steam or H2. From our analysis of V(i) curves for SOFCs with BCY electrolyte, at low H2 concentration, increasing this concentration decreases electronic conductivity somewhat, consistent with adding electrons and reducing hole carrier concentration. At higher H2 concentrations, electronic conductivity remains constant.
Proton transfer in perovskites such as BCY resembles that in usual H‑bonded crystals. Such transfer requires two steps, both needed for dc conductivity. One is intrabond proton transfer, O‑H O→O H-O. The other is a proton jump from one H-bond to an adjacent bond, equivalent to rotation of an O-H unit about the O ion. The main difference from usual H-bonded crystals is that the adjacent O-O pair most likely has no proton between the O ions, so that the Pauling ice rule precluding 2 protons in one bond is not much of a restriction in perovskites because the proton concentration is typically below 0.2 per formula unit. Another difference is that, though protonic semiconductor activation energies are high, in the 0.5 to 1 eV range, the high operating temperatures of SOFCs, 600 to 900 oC, give useful amounts of power per unit area, or in the electrolysis mode, useful amounts of H2 out for steam and electric power input.
Two applications for proton-conducting perovskite ceramics are SOFCs and hydrogen separation membranes (HSMs). For SOFCs, we have modeled the V(i) behavior with almost no adjustable parameters, and have succeeded in coming close to the Nernst open‑circuit potential as well as fitting the V(i) curves for a variety of H2/O2 partial pressure combinations. For HSMs, unlike for SOFCs, an electronic conductivity comparable to the protonic conductivity is desirable, to avoid using a cermet which may crack because of differential thermal expansion.
In conclusion, many oxides and fluorides have O-O or F-F separations in the H‑bonding range and will provide a stimulating playground for further basic and applied research on proton incorporation and dynamics in solids.