No CrossRef data available.
Published online by Cambridge University Press: 22 February 2011
Studies have been conducted at NASA Langley establishing structure-property relationships in polyimides that provide a means of lowering dielectric constants by reducing chain-chain electronic interactions and by incorporation of fluorine into the polymer molecular structure. The resulting polyimides exhibit excellent thermal stability and mechanical strength, improved resistance to moisture and enhanced solubility. Most polyimides containing fluorine in only one monomer are at least partially soluble in common organic solvents, and polyimides containing fluorine in both the dianhydride and the diamine are fully soluble. Although this enhanced solubility aides processability and is desirable for many applications, it is a severe disadvantage for aircraft applications where exposure to hydraulic fluids and other chemicals is likely to occur. This work involves the development of insoluble, low dielectric polymers prepared from 2, 2-bis[4-(3, 4-dicarboxyphenoxy)phenyl]hexafluoropropane dianhydride (BFDA) and selected fluorine-containing diamines.
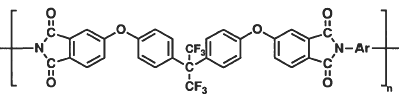
BFDA-containing polyimide films had dielectric constants that ranged from 2.44 to 2.53 at 10 GHz and exhibited insolubility in solvents including N, N-dimethylacetamide, chloroform, diglyme and N-methylpyrrolidinone.