DISCUSSION POINTS
• Battery Recycling – Should battery recycling be internationally mandated as adoption rates increase?
• Recycling methods – Should battery manufacturers incorporate recycling friendly configurations at the expense of cost and energy density?
Introduction
Lithium-ion batteries (LIBs) are often touted to be the key to unlocking renewable energy technologies in global efforts to reduce carbon footprint and human reliance on fossil fuels.Reference Diouf and Pode1 Vast improvements in battery technologies over the past few decades in terms of performance and cost per kWh have resulted in a surge in EV sales and the deployment of large-scale grid storage since 2010.Reference Qiao, Zhao, Liu and Hao2,Reference Harper, Sommerville, Kendrick, Driscoll, Slater, Stolkin, Walton, Christensen, Heidrich, Lambert, Abbott, Ryder, Gaines and Anderson3 Unfortunately, as battery packs from these applications reach their end of life, efforts to incorporate sustainable practices in handling these spent batteries have not yet been well-established.Reference Wang, Gaustad, Babbitt and Richa4 While conventional battery recycling technologies such as pyrometallurgy and hydrometallurgy have been explored, they still face limited adoption in the industry largely due to their energy intensive and costly nature.Reference Wang, Gaustad, Babbitt and Richa4,Reference Li, Zhang, Li, Chen, Wu, Amine and Lu5 Moreover, the use of toxic chemicals during these processes increases the complexity and hazards involved in handling large volumes of spent batteries.Reference Li, Zhang, Li, Chen, Wu, Amine and Lu5,Reference Zheng, Zhu, Lin, Zhang, He, Cao and Sun6 Although recent studies on improved pyrometallurgical and hydrometallurgical methods reported higher metal recovery rates (>95%),Reference Yun, Linh, Shui, Peng, Garg, Le, Asghari and Sandoval7,Reference Lv, Wang, Cao, Sun, Zhang and Sun8 their overall material recovery efficiencies (as a function of the entire cell) remains low due to difficulties in recovering the liquid electrolytes and lithium salts. Crucially, today's batteries are not designed for recycling ease, making it difficult to directly recover the critical materials and embedded value in spent batteries.
To this end, the US Department of Energy's ReCell Center has taken up the mantle, setting out core principles for LIB recycling that involve (i) batteries designed for recyclability, (ii) direct recycling of electrodes, and (iii) recovery of more components within the cell.Reference Spangenberger9 Such guidelines compel researchers and manufacturers to consider battery recyclability beyond material processing or metal recovery and explore means to redesign batteries at the cell to pack level instead, promoting ease of recyclability using cost-efficient and low-carbon footprint processes. However, major battery manufacturers still face difficulties in adjusting existing production protocols and have little incentive to improve current designs especially when profit margins are concerned. As such, it would be judicious for researchers in the field to design robust recycling strategies for next-generation batteries instead, in order to chart pathways for future manufacturers to become early adopters of sustainable production-to-recycling manufacturing processes. Of the various next-generation batteries currently being developed, all solid-state batteries (ASSBs) are regarded to be a highly promising technology that might see widespread applications in electric vehicles and grid storage.Reference Lee, Fujiki, Jung, Suzuki, Yashiro, Omoda, Ko, Shiratsuchi, Sugimoto, Ryu, Ku, Watanabe, Park, Aihara, Im and Han10,Reference Manthiram, Yu and Wang11 Due to their use of nonflammable inorganic solid-state electrolytes (SSEs), wide operating temperature ranges, and potential for high energy density at lower costs per kWh, ASSBs can offer the right balance of factors needed in large device applications. However, there is still a stark lack of studies on ASSB recycling in the literature to date, providing an opportunity to explore possible pathways for recycling ASSBs.
In this work, we propose a sustainable design and scalable ASSB recycling model. This model demonstrates the recovery and regeneration of both the SSEs and electrodes within the cell in order to minimize waste generation and achieve high recycling efficiencies. We conduct life cycle analysis of our recycling design using the EverBatt model and analyze its energy consumption and greenhouse gas (GHG) emissions against conventional recycling technologies. We demonstrate the ability to avoid breakdown of the cell components into their core raw materials, instead directly regenerating them into useful formats for reconstitution. Notably, these are done using safe processing methods without any toxic chemicals or a high carbon footprint. The regenerated materials are then reassembled into a new, fully recycled battery and evaluated against the pristine battery. We show that this process can achieve comparable battery performance to the pristine state and this study provides a promising pathway for large-scale adoption of environmentally friendly and sustainable battery recycling practices.
ASSB recycling model
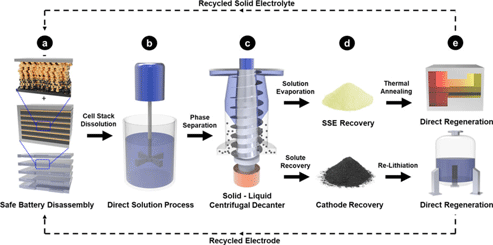
To design a sustainable and practical ASSB recycling model, several criteria need to be met: (i) selection of cell chemistries that allow for efficient component separation with minimal steps; (ii) elimination of toxic, expensive, and low vapor pressure organic solvents; (iii) cost-effective recovery of components in the cell beyond just the cathode; and (iv) processes should be applicable to a variety of cell chemistries.Reference Tan, Banerjee, Chen and Meng12Figure 1 illustrates a proposed five-step model that involves safe cell/pack disassembly, scalable solution processing, component separation, component recovery, and direction regeneration for reuse. For this work, only the sulfide-based Li6PS5Cl (LPSCl) is used as it exhibits a high ionic conductivity (>1 mS/cm), is interface passivating in nature, and has been reported in several studies to demonstrate promise for future commercialization.Reference Lee, Fujiki, Jung, Suzuki, Yashiro, Omoda, Ko, Shiratsuchi, Sugimoto, Ryu, Ku, Watanabe, Park, Aihara, Im and Han10,Reference Nam, Oh, Jung and Jung13 Additionally, sulfide-based SSEs including the commonly reported glassy Li3PS4 or argyrodite Li6PS5X (X = Cl, Br, or I) have been shown to be compatible with scalable solution processing, vital for any successful recycling process.Reference Miura, Rosero-Navarro, Sakuda, Tadanaga, Phuc, Matsuda, Machida, Hayashi and Tatsumisago14–Reference Kim, Oh, Park, Choi, Nam, Lee, Lee and Jung17 Metallic lithium is selected as our representative anode material in this model as its application is widely reported to be the ultimate goal to achieve high energy density ASSBs.Reference Han, Westover, Yue, Fan, Wang, Chi, Leonard, Dudney, Wang and Wang18,Reference Randau, Weber, Kötz, Koerver, Braun, Weber, Ivers-Tiffée, Adermann, Kulisch, Zeier, Richter and Janek19 In this design, fresh lithium metal foil is used at the anode and is assumed to be fully consumed upon reaching the battery's end-of-life. For the cathode, LiCoO2 was used as it is the most common transition metal (TM) oxide-based cathode used in both commercial LIBs as well as ASSBs reported in the literature.Reference Kim, Oh, Park, Choi, Nam, Lee, Lee and Jung17,Reference Zhou, Doerrer, Kasemchainan, Bruce, Pasta and Hardwick20,Reference Gao, Jalem, Ma and Tateyama21 While the Li | Li6PS5Cl | LiCoO2 configuration is used in this study, the processes developed are designed to be applicable to other chemistries as well.

Figure 1. Schematic of the proposed ASSB recycling procedure at an industrial scale, based on the principles of direct recycling. Cell packaging of the ASSB is first removed before the entire cell stack is processed in a solution without further component separation. Solids and liquids are then separated and recovered for direct regeneration via thermal annealing for the solid electrolyte and direct re-lithiation for the cathode.
Compared to commercial organic liquid electrolyte-based LIBs which can pose significant fire hazards during disassembly, the intrinsic nonflammable nature of ASSBs mitigates such safety hazards during the breakdown of large spent battery packs. Upon removal of packaging materials [Fig. 1(a)], no further separation of the cell is required, and the full cell undergoes solution processing using a low-cost, low-boiling point, and safe solvent such as ethanol [Fig. 1(b)]. Previous studies have found that polar solvents such as acetonitrile or various alcohols can induce dissolution of sulfide-based SSEs (that comprise of ![]() ${{\rm PS}_4}^{3-}$ conductive thiophosphate units) and allow recovery into their original chemical state without chemical degradation.Reference Miura, Rosero-Navarro, Sakuda, Tadanaga, Phuc, Matsuda, Machida, Hayashi and Tatsumisago14–Reference Kim, Oh, Park, Choi, Nam, Lee, Lee and Jung17 The dissolution process will result in a suspension of dissolved SSEs in the solution and the spent TM oxide cathodes as the precipitates. The suspension comprising of two phases are then separated using either filtration or gravity-based separation methods such as centrifugal decanting [Fig. 1(c)], followed by drying of the solvent to recover the SSE and cathodes, respectively [Fig. 1(d)]. Upon recovery, the SSEs and cathodes are then directly regenerated, using thermal annealing and chemical re-lithiation, respectively, to produce fully recycled materials that can then be used to assemble new ASSBs [Fig. 1(e)].
${{\rm PS}_4}^{3-}$ conductive thiophosphate units) and allow recovery into their original chemical state without chemical degradation.Reference Miura, Rosero-Navarro, Sakuda, Tadanaga, Phuc, Matsuda, Machida, Hayashi and Tatsumisago14–Reference Kim, Oh, Park, Choi, Nam, Lee, Lee and Jung17 The dissolution process will result in a suspension of dissolved SSEs in the solution and the spent TM oxide cathodes as the precipitates. The suspension comprising of two phases are then separated using either filtration or gravity-based separation methods such as centrifugal decanting [Fig. 1(c)], followed by drying of the solvent to recover the SSE and cathodes, respectively [Fig. 1(d)]. Upon recovery, the SSEs and cathodes are then directly regenerated, using thermal annealing and chemical re-lithiation, respectively, to produce fully recycled materials that can then be used to assemble new ASSBs [Fig. 1(e)].
While the lithium metal anode is used in this recycling design, it is noted that alternative anode materials have been reported as well, such as anode-free, graphite-based, and Li-alloy type configurations.Reference Wan, Kang, Wang, Lee, Zheng, Cui and Sun22–Reference Lee, Fujiki, Jung, Suzuki, Yashiro, Omoda, Ko, Shiratsuchi, Sugimoto, Ryu, Ku, Watanabe, Park, Aihara, Im and Han24 As treatment and separation of unreacted lithium metal are considerably more complex than graphite and metallic alloys, which can be separated using physical methods, using the lithium metal anode in this ASSB recycling design would offer a more conservative approach. In the case where unreacted lithium metal remains, the cell should first be safely discharged to low voltages, ensuring all excess lithium are fully reacted before beginning the recycling process. Alternatively, any trace amounts of lithium remaining can be treated by first preprocessing the cell with heavier alcohols and filtering before the ethanol dissolution step shown in Fig. 1(b). This eliminates the presence of lithium ethoxide impurities within the SSE solution.
Economical and environmental impact analysis
EverBatt model
To evaluate the relative economic and environmental impacts of the ASSB recycling design, the EverBatt model is used to analyze the energy consumption and GHG emissions for various battery recycling processes. Developed by Argonne National Laboratory under the support of the Department of Energy, EverBatt is a publicly available battery recycling cost and environmental impact modeling tool that allows researchers to evaluate the effectiveness of various battery recycling technologies.25 For recycling processes, EverBatt mainly considers pyrometallurgical, hydrometallurgical, and direct recycling routes for both electrolyte and cathode materials. These capabilities are utilized in our ASSB recycling design to evaluate the impact of recycling the SSE and the cathode when compared to conventional LIBs. It is noted that lithium metal recycling is not within the scope of the EverBatt model but could potentially impact overall energy balance considerations. Likewise, while recycling of graphite and other inactive components such as current collectors are possible and should be encouraged, these are out of scope for this work (due to the relatively low economic and environmental impact) and will not be included in the energy and GHG analysis.
Direct cathode recycling
For any new recycling strategy to be effective, it must achieve both lower costs and lower GHG emissions than existing processes. This entails the elimination of sophisticated multi-step processes that are both energy intensive and require handling of toxic organic chemicals commonly seen in hydrometallurgy (Supplementary Fig. S1).Reference Li, Zhang, Li, Chen, Wu, Amine and Lu5,Reference Lv, Wang, Cao, Sun, Zhang and Sun26 Combustion of waste and organics, a core component of pyrometallurgy, should also be avoided to minimize GHG emissions and energy consumption (Supplementary Fig. S2).Reference Li, Zhang, Li, Chen, Wu, Amine and Lu5,Reference Yun, Linh, Shui, Peng, Garg, Le, Asghari and Sandoval27 Thus, direct recycling is a promising alternative for recovery and regeneration of spent battery components. Direct recycling of spent cathode materials has been reported in previous studies using hydrothermal re-lithiation or molten eutectic salts to directly regenerate degraded electrodes to their pristine states without the breakdown of their core chemical structures (Supplementary Fig. S3).Reference Shi, Chen and Chen28–Reference Liu, Zhang, Chen, Lin, Zhang and Lu30Figure 2(a) compares the relative energy consumption (energy needed to recycle 1 kg of spent LiCoO2) of direct recycling compared to pyrometallurgy and hydrometallurgy. The energy required to directly regenerate cathodes is 78% lower than pyrometallurgical and 86% lower than hydrometallurgical methods, respectively. It is important to point out that these energy values also account for consumption during upstream material processing such as the production of chemicals required for recycling. The large differences in the total energy required stems from a reduced material input during regeneration as major inputs in direct methods only involve lithiation precursors such as LiOH and Li2CO3. In contrast, large volumes of acids (such as H2SO4) required in the leaching steps used in hydrometallurgy, and heat energy used in smelters during pyrometallurgy, are major contributors to the high energy usage in these processes. Consequently, this has a direct impact on the amount of GHGs released as seen in Fig. 2(b). Although GHG output from pyrometallurgical recycling is the highest among the three methods compared (mainly due to the high combustion output during smelting that releases large amounts of exhaust gas and flue dust), the total amount of GHGs released via hydrometallurgy is merely 3.3% lower. The high amount of GHGs from the materials input component is a result of large amounts of acids needed for leaching (Table 1). While the emissions do not come from the leaching process itself, upstream production of GHGs such as CO2 and SOx during the manufacturing of sulfuric acid is the main contributor toward GHGs (Table 2). Conversely, direct recycling results in approximately 1/5 of the GHG emissions compared to conventional methods, due to the absence of material or energy-consuming processes and nondestructive regeneration methods. However, direct recycling methods also require more delicate sorting processes based on their respective electrode chemistries such as commercially used LiCoO2, LiFePO4 (LFP), Li(NixMnyCoz)O2 (NMC), Li(NixCoyAlz)O2 (NCA) (x + y + z = 1), or other cathode materials. This can be challenging to achieve for third party recyclers who may not have access to complete information on cell chemistries from the original battery manufacturers, making it difficult to both separate and select the appropriate direct regeneration conditions to recycle spent materials. Additionally, direct recycling methods reported in the literature often includes only the cathode, while the other cell components are discarded or not treated. Until more components of the cell are recovered, the recycling efficiency as a function of the entire cell will remain low.

Figure 2. Energy and environmental impact analysis from upstream processing to the fully recycled state. (a) Total energy consumption and (b) GHG emission comparisons from direct methods, conventional hydrometallurgy, and pyrometallurgy for LiCoO2 recycling. (c) Energy consumption and (d) GHGs emission comparisons between solid and liquid electrolyte recycling in full cells using solution processing with heat treatment and supercritical CO2 extraction, respectively.
Table 1. Materials requirements to recycle 1 kg of spent batteries via different recycling technologies.

Table 2. Total emissions and breakdown of GHGs to recycle 1 kg of spent batteries via different recycling technologies.

Electrolyte recycling
To address these concerns, recent studies have explored the recovery of lithium within the organic liquid electrolytes and salts using supercritical CO2 extraction, allowing a greater fraction of the spent LIB to be recycled (Supplementary Fig. S4).Reference Nowak and Winter31–Reference Grützke, Mönnighoff, Horsthemke, Kraft, Winter and Nowak33 As electrolytes, salts and additives typically make up 10-15% (weight fraction) of the entire cell,Reference Li, Zhang, Li, Chen, Wu, Amine and Lu5, Reference Golubkov, Fuchs, Wagner, Wiltsche, Stangl, Fauler, Voitic, Thaler and Hacker34 their recovery in combination with direct recycling of cathode materials (which typically makes up 25–40% of a cell)Reference Li, Zhang, Li, Chen, Wu, Amine and Lu5,Reference Golubkov, Fuchs, Wagner, Wiltsche, Stangl, Fauler, Voitic, Thaler and Hacker34 offer a promising strategy to harvest a major fraction of the valuable components in spent LIBs and reduce waste generation at the same time. In principle, the enhanced dissolution properties between the liquid and gaseous phase of supercritical CO2 allows high-yield extraction of organic substances along with any dissolved salts, enabling recovery rates up to 90% of the liquid electrolytes.Reference Liu, Mu, Zheng and Dai32,Reference Grützke, Mönnighoff, Horsthemke, Kraft, Winter and Nowak33 However, due to the additional facilities required to maintain the temperature and pressure conditions, processing energy costs will increase slightly [Fig. 2(c)]. Nonetheless, overall energy costs and GHG emissions from liquid electrolyte recovery and direct recycling of spent LIBs are still significantly lower than traditional pyrometallurgy and hydrometallurgy methods (Table 2).
In our ASSB recycling design, SSE recovery and regeneration are incorporated into the EverBatt model (Supplementary Fig. S5). To recycle Li6PS5Cl (used in our example), ethanol is employed to dissolve and precipitate the SSE from the composite electrode and separator layers as seen in Fig. 1. Despite the need to overcome vaporization enthalpies to evaporate and recover ethanol, its high vapor pressure compared to common organic solvents (such as N-Methyl-2-pyrrolidone) significantly reduces the energy requirements needed for processing. This translates into marginal increases in the corresponding emissions compared to when only the cathode is recycled [Fig. 2(d)]. GHG emissions from ASSB recycling stems from mainly CO2, due to electricity use during processing (generated from fossil fuels). The amount of energy required can be further reduced if ethanol is evaporated under vacuum conditions without the use of any heat, provided ambient temperatures are sufficiently high (>20 °C) for vaporization. Although not considered in this model, condensation enthalpies can also be reclaimed in large-scale industrial processes during ethanol recovery for reuse. Despite the fundamentally different cell chemistries of ASSBs versus LIBs used in this study, the energy and environmental analysis arising from 1 kg of spent batteries shows the importance of adopting direct recycling methods to lower costs as well as GHG emissions across both types of cells. Furthermore, the incorporation of electrolyte recycling can dramatically improve recycling efficiency as a function of the entire cell, notably with only marginal increases in energy and environmental costs, making it an effective strategy to handle both spent LIBs and ASSBs at their end of life.
Results and discussion
To demonstrate the feasibility of our ASSB recycling model, the structural and electrochemical properties of the SSE and cathode at both the pristine and fully recycled states were experimentally evaluated. As ASSBs are not currently commercially available, pristine ASSBs were fabricated and subsequently recycled after a certain number of defined cell cycles. For this study, Li | Li6PS5Cl | LiCoO2 full cells were assembled and tested at room temperature for 100 cycles before application of the direct recycling strategy. After recovery and regeneration of the SSEs and cathodes, these recovered materials will be reassembled into ASSBs (fully recycled ASSBs) and compared against their pristine states in order to evaluate the efficacy of the recycling design.
Li6PS5Cl SSE recovery and regeneration
Fundamentally, spent bulk SSEs in both the separator layers and cathode composites do not undergo significant chemical degradation even after prolonged cell cycling, with the exception of minor decomposition at the cathode interface.Reference Tan, Wu, Nguyen, Chen, Marple, Doux, Wang, Yang, Banerjee and Meng35,Reference Auvergniot, Cassel, Ledeuil, Viallet, Seznec and Dedryvère36 As a result, most of the SSE can be directly recovered without sophisticated re-synthesis. After dissolution in ethanol and precipitation, the recovered SSE was found to exhibit an ionic conductivity (Table 3) of about 1 order of magnitude lower (0.11 mS/cm) than its pristine state (1.62 mS/cm). This was reported in previous studies to be due to reduced grain sizes and a poor degree of crystallinity in recovered SSEs rather than a result of chemical degradation against the organic solvents used.Reference Kim, Oh, Park, Choi, Nam, Lee, Lee and Jung17 Reductions in particle size can also be observed after the dissolution process [Figs. 3(a) and 3(b)]. The recovered Li6PS5Cl was then heated under vacuum and characterized with X-ray diffraction (XRD), Raman spectroscopy, and electrochemical impedance spectroscopy (EIS). From Figs. 3(c) and 3(d), both the bulk and local structures of recycled Li6PS5Cl were recovered after the direct regeneration process. An ionic conductivity of 1.48 mS/cm was measured after recycling, which is within the same order of magnitude of its pristine form [Fig. 3(e)]. Thus, thermal annealing was demonstrated to be effective in regaining the pure phase and the high ionic conductivity of solution-processed SSEs.

Figure 3. Li6PS5Cl particles at the (a) pristine state and (b) recycled state. The average particle size of Li6PS5Cl decreased after the solution process. Characterizing Li6PS5Cl solid electrolyte at the pristine and regenerated state. (c) XRD patterns showing the retention of the bulk structure. (d) Raman spectra demonstrating the retention of local thiophosphate units. (e) Nyquist plots from impedance measurements indicate the retention of ionic conductivity.
Table 3. Ionic conductivity of the Li6PS5Cl solid electrolyte and ICP results of the LiCoO2 cathode materials at the pristine, cycled, and regenerated states.

Ionic conductivity was measured via electrochemical impedance spectroscopy measurements.
LiCoO2 cathode direct recycling
After the phase separation steps described in Fig. 1, spent cathodes were recovered as precipitates. Using inductively coupled plasma mass spectrometry (ICP-MS), it was found that spent LiCoO2 contains depleted Li+ amounts (Table 3) compared to the pristine cathode, which has been typically reported on cathodes harvested from cycled conventional LIBs. However, unlike liquid electrolyte-based LIBs, LiCoO2 cathode particles from ASSBs would also contain cathode electrolyte interphase (CEI) products deposited on the surface as a result of SSE oxidation during cell cycling [Fig. 4(a)]. This CEI layer needs to be treated and removed before direct regeneration can be applied to re-lithiate the LiCoO2 particles. As previous studies have found that the oxidized products of Li6PS5Cl mainly comprise of elemental S, P2S5, and LiCl,Reference Tan, Wu, Nguyen, Chen, Marple, Doux, Wang, Yang, Banerjee and Meng35,Reference Auvergniot, Cassel, Ledeuil, Viallet, Seznec and Dedryvère36 all of which are soluble in or can be physically removed with water, the recovered cathode was surface treated with water before hydrothermal regeneration. It is noted that an LiNbO3 coating is typically used in cathodes for ASSBs to avoid chemical reactions with SSEs; this coating material is inert to water and is retained after the recycling process. After hydrothermal re-lithiation, ICP measurements found that LiCoO2 regained its original lithium content and thus became fully regenerated. While solid-state sintering using suitable lithium sources may be equally effective to directly regenerate the cathode, such methods require accurate quantification of its state of decay and lithium source ratio in order to avoid depositing impurities onto the regenerated cathodes. This may be difficult to achieve on the commercial scale where spent batteries from different devices and sources are obtained. Thus, the hydrothermal method would be a more robust method that can be applied across cathodes harvested from different cells.

Figure 4. (a) Schematic of the LiCoO2 cathode surface treatment and regeneration process. (b) Voltage profile of the Li | Li6PS5Cl | LiCoO2 cell in the pristine and recycled state, with the schematic of the cell setup in the inset. (c) Cycle performance of the Li | Li6PS5Cl | LiCoO2 cell in the pristine and recycled state. Cells were cycled at room temperature, under a stack pressure of 5 MPa, and at a rate of 0.1C. The typical active mass loading was 10 mg/cm.
Electrochemical performance
To evaluate each recycled component, both regenerated Li6PS5Cl and LiCoO2 were used to fabricated new ASSBs using fresh lithium metal foil and cycled under similar conditions as the original cell. Figure 4(b) compares the 1st cycle voltage profile of the pristine and the recycled cell. Both cells displayed comparable 1st cycle charge and discharge capacities as well as overall cell polarization, with slight differences which can be attributed to temperature fluctuations. Cell stack pressures of 5 MPa were used for cycling as it was previously found to enable long cycle life of lithium metal ASSBs.Reference Doux, Nguyen, Tan, Banerjee, Wang, Wu, Jo, Yang and Meng37Figure 4(c) shows the capacity retention as well as Coulombic efficiencies with extended cell cycles. Initial capacity fade in both cells is attributed to mechanical contact losses between the SSE and the cathode, typical of ASSBs with similar cell configurations. After the initial capacity loss, both cells achieved high capacity retention and average Coulombic efficiencies of >99.9% after the 5th cycle [Fig. 4(c)]. While these results demonstrate the effectiveness of recycling the spent ASSBs, this has yet to be tested in a full cell pack with typical commercial-sized capacities (>2 Ah). Thus, it is not clear how multilayer stacked cells (that may contain carbon additives, binders, and other additional components) may influence the recycling approach. Thus, processes would need to be optimized and adjusted to suit the cell configurations of future commercialized ASSBs.
Nonetheless, the recycling principles of separation, recovery, and direct regeneration can also be applied to alternative cell chemistries. Other types of SSEs can be processed with inexpensive and relatively safe solvents such as acetonitrile, water, methanol, and other organic solvents that have high vapor pressure.Reference Banerjee, Park, Heo, Nam, Moon, Oh, Hong and Jung38,Reference Li, Liang, Chen, Luo, Adair, Wang, Banis, Sham, Zhang, Zhao, Lu, Huang, Li and Sun39 This allows cathode materials to be separated from the dissolved SSEs during recovery. Likewise, direct regeneration methods can be applied to alternative cathodes as well, using either solid-state sintering methods or molten eutectic salts discussed earlier, to enable direct re-lithiation of the NMC cathode (harvested from spent LIBs) under ambient pressure conditions.Reference Shi, Zhang, Meng and Chen29,Reference Song, Hu, Liang, Long, Zhou, Song, You, Wu and Liu40 As spent batteries are generally defined by a 20% loss of reversible capacity, most of the materials within any spent cell should still be in usable condition and thus require only mild regeneration to regain their pristine properties. While lithium metal anodes are consumed in this model, the principles of separation, recovery, and regeneration can also be applied to other types of anodes such as conventional graphite or next-generation silicon anodes as well. Ultimately, the direct regeneration of cathodes and recovery of electrolytes are an important and promising method to reduce both energy consumption and GHG emissions toward a long-term and sustainable battery recycling strategy.
Conclusions
In this study, a sustainable next-generation ASSB design and recycling strategy was introduced. This approach demonstrates the recovery and regeneration of SSEs and cathodes from spent batteries without using toxic chemicals or energy-intensive processes. Considerations for anodes were also discussed. The EverBatt model was employed to evaluate the energy consumption and environmental impact of the recycling strategy and compare it with traditional pyrometallurgical and hydrometallurgical methods. It was found that direct recycling methods significantly reduce both energy consumption and GHG emissions as a result of reduced material requirements from upstream processing as well as eliminating the need for smelting. Moreover, techniques to recover the electrolyte in spent ASSBs were shown to only slightly increase the energy consumption and emissions. To validate the model, pristine Li | Li6PS5Cl | LiCoO2 full cells were experimentally fabricated and regenerated. Regenerated Li6PS5Cl was found to have similar structural properties and ionic conductivity compared to pristine Li6PS5Cl and LiCoO2 was able to regain the lithium content lost during cell cycling. The fully recycled solid electrolyte and cathode materials were reassembled into a full cell and demonstrated similar electrochemical properties and capacity retention compared to the pristine cell. The results shown here demonstrate the feasibility of direct recovery and regeneration of SSEs and cathodes in next-generation ASSBs of various chemistries, offering a scalable, low-cost, and sustainable pathway for handling spent batteries at their end of life.
Acknowledgments
Z.C. gratefully acknowledges funding from US National Science Foundation via Award CBET-1805570 and the start-up fund support from the Jacob School of Engineering at UC San Diego. Y.S.M. acknowledges the funding support from Zable Endowed Chair Fund.
A patent was filed for this work through the UCSD Office of Innovation and Commercialization.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1557/mre.2020.25.