DISCUSSION POINT
• This work aims to initialize a discussion on “if” and “how” the recent and unprecedented lead halide perovskites can enter the consumer market.
Introduction
Over the past decade, metal halide perovskites have been found to possess tremendous potential for optoelectronic applications.Reference Zhang, Eperon and Snaith3–Reference Kojima, Teshima, Shirai and Miyasaka6 Despite crystalline silicon being the prevailing photovoltaic technology to date, photovoltaic perovskites that are generally described by the generic formula ABX3 (with A = CH3NH3+, HC(NH2)2+, Cs+, and/or Rb+; B = Pb2+ and/or Sn2+; X = I−, Cl−, and/or Br−) have garnered considerable interest owing to their exceptional intrinsic properties, such as ambipolarity, high charge-carrier mobilities, high diffusion lengths, and high absorption coefficients.Reference Lee, Teuscher, Miyasaka, Murakami and Snaith7–Reference Stranks, Eperon, Grancini, Menelaou, Alcocer, Leijtens, Herz, Petrozza and Snaith10 Marking the start of worldwide and intensive interdisciplinary research efforts, impressive advancements in material composition, film formation, and interface as well as device engineering have pushed metal halide perovskite power conversion efficiencies beyond the level of CdTe, CIGS and multicrystalline silicon. Accordingly, the performance of research cells using these light harvesters has swiftly increased from the seminal 3.8% reported in 2009 to an impressive certified record efficiency of 22.7%—ranking metal halide perovskites as the fastest-growing photovoltaic technology to date.Reference Kojima, Teshima, Shirai and Miyasaka6,11,Reference Brenner, Egger, Kronik, Hodes and Cahen12 Unlike the stringent manufacturing process of traditional silicon photovoltaics, metal halide perovskites profit from a simple and cost-effective solution-based deposition, offering the additional benefit of serving a wider range of innovative applications as it renders them highly flexible and lightweight. Moreover, in view of their tuneable band gap, these efficient absorbers are also remarkably satisfying in terms of aesthetics, as they can be fabricated in a wide variety of colors while maintaining substantial efficiencies. In addition, the tuneable absorption window also enables the absorbers to be implemented in tandem devices that in turn allows more light to be harvested from the solar spectrum. As such, it is clear and undeniable why photovoltaic perovskites are quickly gaining commercial interest. In light of the current need for sustainable energy resources, several start-up and spin-off companies have been established, initially promising modules on the market by the end of 2017.13–Reference Van Noorden15 Nevertheless, the heavy metal content of this promising technology is enough reason to become wary of its large-scale implementation. Lead representing approximately one third by weight of the most archetypical absorber layer has raised considerable concern with respect to the potential environmental impact of the technology.Reference Babayigit, Thanh, Ethirajan, Manca, Muller, Boyen and Conings16–Reference Babayigit, Ethirajan, Muller and Conings18
Since its discovery around 7000 B.C., lead has found purpose in diverse and numerous aspects of preceding primary civilisations.Reference Stos-Gale and Gale19,Reference Gale and Stos-Gale20 Desired for its versatile material properties such as high density, low melting point, malleability, ductility, high resistance to corrosion (thereby neglecting a thin reacted surface layer), and propensity to react with organic reagents, the heavy metal has become of high significance in prevalent applications and evolved to become indispensable to our present-day society.Reference Haynes21–Reference Guruswamy23 Having its terrestrial origin in space as a result of neutron capturing (at supernovae explosions), lead is positioned at the end of three major decay chains of newly formed heavier elements. One of the oldest and most-refined radiometric dating schemes is based on uranium-lead decay. Remarkably, this allowed perhaps one of the most peerless yet unheard-of applications of lead—the first accurate determination of the age of the Earth.Reference Patterson24 Rarely found in its native, metallic form, lead is generally found in the form of its principal ore galena (PbS).Reference Gale and Totemeier22,Reference Davidson, Ryman, Sutherland, Milner, Kerby, Teindl, Melin and Bolt25 Being a relatively light weighted mineral, this ore did not collect deeper at Earth’s core, resulting in a relatively high crustal abundance of lead (14 ppm)—its presence at Earth’s surface (soil) and atmosphere being due to the ever-increasing anthropogenic lead mining.Reference Patterson, Ericson, Manea-Krichten and Shirahata26,Reference Flegal and Smith27 As a result, galena being earth-abundant and easy to mine makes that lead is relatively inexpensive and an economically relevant metal. Nonetheless, lead is known to be hazardous to human health and the environment, and therefore—despite its numerous favourable traits—many wonder where the acceptable boundaries lie regarding its use.Reference Babayigit, Ethirajan, Muller and Conings18,28–Reference Cullen and Kolev30 Especially considering the past five decades, during which the detrimental long-term effects of chronic lead exposure have been rigorously demonstrated, lead in consumer products has become the focal point of many discussions. Despite lead compounds generally being sparingly soluble in water, it is their increased solubility in acidic solutions that provides a considerable and alarming pathway of bioaccumulation.Reference Clever and Johnston31 Currently, listed as one of the ten chemicals of major health concern by the World Health Organisation, its use has become prohibited in some industrial and domestic applications to prevent occupational and non-occupational exposure.29,32 The metal and its compounds, which are, moreover, classified as carcinogenic, can inflict serious acute and chronic damage to virtually all organs and tissues of the body, particularly to the nervous system. Exerting more pronounced neuronal deficits in children, due to their vulnerable stage of on-going development, and resulting in their life-long intellectual impairment, it has now been established that no threshold value can be indicative of intoxication as any detectable amount of lead in the blood is considered detrimental for human health.
Despite the photovoltaic community being aware of the toxicity associated with lead, the significant lead content of metal halide perovskites is imposing a great strain on their public perception and acceptance. The magnitude of misgivings expands even more as recent studies also demonstrate successful application of metal halide perovskites in light emitting diodes, lasers, batteries, and photodetectors.Reference Zhang, Eperon and Snaith3–Reference Stranks and Snaith5,Reference Sutherland and Sargent33 Hence there is no doubt that a discussion should be commenced on how to assess and handle the impact of lead content in a new technology of such high potential. Presently, the use of lead in consumer products is governed by rigorous regulations either restricting the use of hazardous substances or striving for recyclable products to mitigate any further increase in environmental lead accumulation.34,35 As a result, an astonishing 11,144,000 tons of lead have been produced worldwide in 2016, of which only 4,721,000 tons account for lead extracted from primary resources (i.e., lead mining).2 The remaining 58% was extracted from secondary sources in which lead is mainly recovered from recycling products or production residues.2 In the United States, more than 80% of lead comes from secondary production, whereas in Europe this amounts to 60%.36 In spite of these impressive figures, it remains challenging to carefully screen existing and novel applications for hazardous substance employment. Taking into account the exclusions and exemptions to the standing directives, and above all considering their rather recent implementation at the beginning of the 21st century (meaning that these policies are still taking shape today), compliance does not unambiguously imply that lead is used in an environmentally justified manner.
Considering the critical role of lead in industrial and technological development, careful scrutiny on directives governing lead consumption is becoming increasingly relevant for the unchallengeable exploitation of lead halide perovskites in optoelectronic applications. Acknowledging that current legislation is shaped by our historical experiences with lead, this work devotes a substantial section to a comprehensive and well-supported overview on anthropogenic lead poisoning. Based on this, we then focus on the legal content of worldwide directives and carefully reflect on their implications regarding lead halide perovskite commercialization. Ultimately, this work aims to initialize a discussion on “if” and “how” the recent and unprecedented lead halide perovskites can enter the consumer market.
History of anthropogenic lead poisoning: the continuing lead blindness
Here, a chronological and comprehensive summary is provided that highlights key events with respect to the history of anthropogenic lead poisoning since antiquity. As outlined in boxes A–F (supplementary information), lead was a chief metal in human history. A statuette found in Asia Minor dated at 6500 B.C. is one of the oldest artifacts corroborating its ancient and extensive use.Reference Stos-Gale and Gale19,Reference Gale and Stos-Gale20 Minted coins linked to ancient Chinese civilisations dated at 4000 B.C. indicate that lead was neither a stranger to the ancient Far East.Reference McKetta37
Antiquity (800 B.C.–500 A.D.)
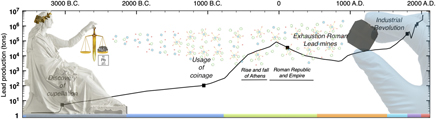
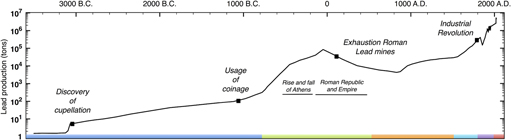
As indicated in Fig. 1, the first prolific use of lead dates back to the Roman Empire. Peaking at an estimated 80,000 tons of lead mined per year, the Romans conducted the first mass production of lead, 3000 years after cupellation was invented.Reference Hong, Candelone, Patterson and Boutron1 As detailed in box B, lead found applications in diverse and vital aspects of Roman civilisation, and while its biocidal properties were recognised and recorded as early as 2000 B.C., liberal use of lead was made for better or for worse.Reference Gilfillan38–Reference Nriagu42 The first correlation between lead and its adverse health effects was established in Therica and Alexipharmaca by Hellenistic philosopher Nikander of Colophone (250 B.C.).Reference Colophonius, Schneider and Keil43 He reported on the acute effects of lead poisoning, such as colic, anemia, and paralysis, associated with high-dose exposure. Ahead of his time, Greek physician Hippocrates of Kos (450–380 B.C.)—referred to as the father of modern medicine—was among the first to relate gout to food and wine, albeit the association between gout and lead poisoning was not yet established at that time.Reference Waldron44 Among the Romans, civil and military engineer Vitruvius (80–15 B.C.) is cited as one of the earliest sources reporting on plumbism. Together with Pliny the Elder (23–79 A.D.), who wrote Naturalis Historia, he was among the first to recognise the hazard of lead fumes and its impact on slaves manufacturing it in mines and smelters.Reference Waldron44,Reference Secundus and Hardouin45 In De Architectura, he aptly corroborates the latter by correlating the adverse effects of white lead [2PbCO3·Pb(OH)2] and water from leaded aqueducts “Water conducted through earthen pipes is more wholesome than that through lead. Indeed that conveyed in lead must be injured, because from it white lead is obtained, and this is said to be injurious to the human system. This may be verified by observing the workers in lead, who are of a pallid color. For casting lead, the fumes from it fixing on the different members, and daily burning them, destroy the vigor of the blood”.Reference Kruft46 As a conclusion, he stated “Water should therefore on no account be conducted in leaded pipes if we are desirous that it should be wholesome”.Reference Kruft46 Instead, he recommends the use of earthen pipes, except his warnings went unheeded.

Figure 1. Timeline world lead mining (logarithmic scale). Before the 1700s, the most prolific use of lead dates back to antiquity and the Roman Empire reaching annual lead production of 80,000 tons/year. The massive scale of Roman lead mining and smelting declined after the fall of the Western Roman Empire and was not matched until the Industrial Revolution. The color code in the timeline matches each period and its corresponding box in the supplementary information. This graph has been compiled from Refs. Reference Hong, Candelone, Patterson and Boutron1 and 2.
An estimated 12,000 tons of lead were used in the aqueduct at Lyons, and as lead pipes and lead seals on clay pipes were used extensively in plumbing, and aqueducts and reservoirs were lined with sheet lead, many distinguished historians now believe the resulting enormous lead intake hastened the fall of the Roman Empire.Reference Gilfillan38–Reference Nriagu40 However, according to De Aquaeductu, written by Roman senator Frontinus (40–103 A.D.), calcium carbonate (CaCO3) deposits (resulting from natural processes) protected the aqueducts against corrosion with the additional benefit of insulating against any leaching of lead.Reference Rodgers47 In addition, with no taps on the flow, water flowed continuously and would therefore never have been in prolonged contact with the metal lining even if it were bare. The above implies that the high osteological lead levels in Roman remains cannot solely be attributed to lead plumbing as moreover has been corroborated by Delile et al. (2014).Reference Delile, Blichert-Toft, Goiran, Keay and Albarède48 Based on lead isotope measurements on Roman sediment cores, the authors demonstrated that despite Roman ‘tap water’ containing 100 times more lead than local spring water, the measurable lead pollution could have not been harmful enough to be the prime culprit of Rome’s demise. Rather than CaCO3–encrusted lead pipes, the use of Sapa, Carenum, and Defrutum accounted for the chronic plumbism observed in Roman remnants.Reference Nriagu39,Reference Nriagu40 As described in box B, the Romans had a taste for lead, as they preferred sweet Sapa in their wines and meals. Austrian physician Eisinger (1924) determined an astonishing 1 g of lead per liter in the concentrated aromatic syrup he prepared in 1883 following the Roman recipe by Columella.Reference Eisinger49 With our current knowledge on plumbism, one can say that one teaspoon of sapa would have been enough to cause chronic lead poisoning.29 Pharmacologist Dioscorides (40–90 A.D.), author of De Materia Medica, writes “Generally, all unmixed and simple wine (hard by nature) is warming, easily digested, and good for the stomach. It encourages the appetite, is nourishing, induces sleep, and causes a good color. Those with sapa, however, fill the head causing drunkenness.” testifying to the awareness of the adverse effect of adulterated wine.Reference Dioscorides and Sprengel50 In the late Roman period, gout prevailed among the aristocrats including most of the emperors (e.g., Claudius, Caligula, and Nero).Reference Nriagu39,Reference Nriagu40 Taking into account the vast employment of lead in Roman society, and the current knowledge on lead-induced nephropathy, it is now believed that Roman gout resulted from enormous lead intake, especially that associated with the aristocratic lifestyle. Considering the rampant lead-tainted Roman diets and the water transported in leaded aqueducts along with all other pathways of lead in Roman society, the Roman lead intake is estimated to have varied from 35 to 250 mg/day compared to 0.3 mg daily intake established by the National Academy of Sciences in 1980.Reference Needleman51 With our present-day knowledge that any measurable amount of lead may have detrimental health effects, the role of lead in the decline of the Roman Empire is likely but still under debate.
At that time, the effects of lead were either offered a natural explanation by elite healers such as Hippocrates or countless spiritual explanations by diviners and priests. A divine explanation that has outlasted many saecula and still remains in use today is that of Saturnus. The Romans, who named the planets after their Gods based on planetary motion, named their farthest planet after Saturnus, Saturn. Saturnus was the God of many things, above all ‘King of the Gods’ who represented authority, discipline, and hard work. Having eaten his own children and having castrated his own father, he was also considered a rather melancholic, sullen, and ominous character. Moreover, being the ‘God of time’, he was associated with the slow orbit of Saturn that at that time marked the vast boundaries between human existence and the infinite and unknown space beyond. Testing the limits of man, lead’s deteriorating and retarding neuropathic sequelae were soon associated with the firm reign of Saturnus and the slow orbit of Saturn. Wrongly thinking that moderate exposure to lead would do no harm, Saturnine karma would befall those who dared to challenge Saturnus.Reference Nriagu39,Reference Finger52 As dense, dark, and heavy as the metal itself, he would be named the ‘God of lead’, and many applications of lead thereafter would be named Saturnine, such as in Saturnine drugs (boxes A and B), Saturn’s sugar (box B, lead acetate [Pb(CH3COO)2]), and the spirits of Saturn (boxes B and D, white lead, or Venetian ceruse).
Overall it can be stated that in spite of the initial Roman nescience on plumbism, years of experience and observations brought them closer in understanding and correlating detrimental health effects to lead exposure. It was recognized and recorded both on an occupational level and at an exceptionally high non-occupational level among aristocrats. Whilst cautioned by many great and respected figures such as Nikander, Vitruvius, and Dioscorides, warnings went unheeded, resulting in endemic chronic plumbism. After the fall of the Western Roman Empire, the massive scale of Roman lead mining and smelting was not matched until the Industrial Revolution.
Middle ages (500–1500)
Entering the Middle Ages, the use of lead declined in Europe with its production not being revived until the 11th and 12th centuries.Reference Hong, Candelone, Patterson and Boutron1,Reference Gimpel53 Initially recycled from abandoned Roman cites, lead mining picked up prompting a marked increase in the use of lead-containing products.Reference Winder54,Reference Rich55 Considering the preceding Roman awareness on plumbism, it would astoundingly anew become the illness of patricians who mainly resided in urban areas.Reference Rasmussen, Skytte, Jensen and Boldsen56 As indicated in box C, elaborate goblets, plates, and other cooking ware that the privileged ate from were glazed using lead. Upon ingesting salty and acidic foods served on them, the lead-based glaze would partially dissolve, having lead seep into meals and ultimately find its way to the body. As those living by the countryside could only afford unglazed pottery, their rural uptake of lead was less compared to townspeople, as corroborated with osteological evidence by Rasmussen et al. (2015).Reference Rasmussen, Skytte, Jensen and Boldsen56 However, glazed pottery was not the only source of lead in the medieval period. Lead was ubiquitous and it was also present in coins and stained glass windows and remained in use in plumbing, as evidenced by lead tiles found on roofs.Reference Blair, Blair and Ramsay57 It is believed that at that time drinking water was collected via the roof and therefore contained substantial amounts of lead that similarly to the Roman period contributed to lead poisoning. Furthermore, as lead played an imperative role in glass manufacturing, its adverse fumes liberated during production and glazing additionally opposed a great occupational threat to the medieval unprivileged just as it did to Roman plebeians.Reference Warren58 In like manner, lead dust liberated when cutting lead cames represented a significant pathway of occupational exposure, especially when considering the vast amount of imposing stained glass works of the medieval period. However, development of concern for occupational and environmental health was not yet deemed important at that time, as long as cheap labor was available. Despite ancient publications and ominous writings of medieval physicians on plumbism, warnings remained disregarded and lead and its compounds endured widely in domestic and industrial applications, among them lead sugar embodying the pathway of the largest detrimental burden.Reference Eisinger49,Reference Needleman51 In spite of cane sugar being widely known and distributed by the end of the medieval period, its high price and exclusivity urged many to continue the use of Saturnine sugar or sapa for the sweetening of meals, wines, ciders, and other beverages.Reference Eisinger49 Inflicting a consecutive mass poisoning comparable to that recorded in the Roman period, the first edicts banning lead would emerge by the end of the medieval period. Recognizing the dangers of fortified wines, both the French and Spanish authorities issued edicts in 1427 prohibiting the addition of sapa to sweeten wines.Reference Lessler59 In 1478, the German authorities even made wine adulteration a crime punishable by the death penalty. By 1494, a papal bull forbade the use of fortified wines as it was deemed unsuitable for sacred rites, nonetheless adulterated wine continued to be consumed progressively.Reference Winder54
Early modern period (1500–1789)
Into the early modern period, the lead blindness continued despite the comprehensive studies published on the occupational hazards of lead by Ellenbog (1435–1499), Von den giftigen besen Temppfen Reuchen der Metal, and Agricola (1495–1555), De Re Metallica.Reference Metzler60,Reference Hoover and Hoover61 Toward the 17th century, a series of severe outbreaks of colic were reported in Southern Germany, with similar reports dating from antiquity. Only by 1696, the correct etiology of the disease was discovered by German physician Gockel (1636–1703) during a severe outbreak of colic at the local monastery of Ulm.Reference Eisinger49 The monastery, being a closed community, provided an ideal setting for Gockel to establish that the litharge (PbO) added to sweeten the wine was the culprit of the most widespread anthropogenic pandemic in human history. Resulting from a decree issued by Duke Ludwig of Württemberg in 1696, lead-based additives became forbidden in wine.Reference Eisinger49 Interestingly, the decree—offense being punishable with the death penalty—was not invoked due to environmental or health concerns, but rather on economical grounds to preserve the reputation of German wine.
Swiss physician Tronchin (1709–1781) later published a detailed work profoundly explaining the causes of lead colic entitled De colica pictonum.Reference Tronchin62 Notably, only by 1713, Italian physician Ramazzini (1633–1714) linked the disease shared by potters, guilders, glassmakers, and metal workers to plumbism in his famous work De Morbis Artificum Diatriba.Reference Ramazzini63 Despite the plethora of literature on diverse aspects of lead poisoning, worldwide plumbism persisted growing monotonously. Due to the Treaty of Methuen that England signed in 1703, fortified wines from Spain and Portugal continued to be produced and imported.Reference Lessler59 As a result, widespread lead poisoning and a serious gout epidemic had risen among the English upper class. Atomic absorption spectroscopy analysis of English port wine bottled during the period of 1770–1830 indicated contents up to 1900 mg of lead per liter, being roughly twice as concentrated as Roman sapa.Reference Nriagu40 Composer van Beethoven (1770–1827), known to be a heavy wine drinker, suffered from elevated lead levels—as was detected in his hair remnants—now raising controversy in his death that potentially might have been hastened by lead poisoning.Reference Mai64 Nevertheless, wine was not the only pathway of lead intoxication. The Devonshire colic, studied by Baker (1722–1809), was yet another colic outbreak caused by the use of leaded parts for making apple cider.Reference Barry and Mossman65 In 1745, Franklin (1706–1790) communicated on the enteric and neuropathic effects of drinking lead-laced rum and other spirits in Essays on West India Dry Gripes.Reference Barry and Mossman65 He also writes about the ‘dry gripes’ (colic) and ‘dangles’ (wrist drop) that affected tinkers, painters, and typesetters. In the same colonial period, in 1723, the Massachusetts Bay Colony passed an act prohibiting the distillation of rum and other strong liquors with leaded covers and pipes while, in contrast, lead oxide and lead acetate remained in use to whiten and sweeten bread, alongside with red lead used as a pigment in red pepper.Reference Lessler59
Late modern period (1789–1945)
In the dawn of the industrial revolution, lead mining rates spiked, exceeding those of the great Roman Empire. As detailed in box D, lead-based pigments became of vast demand in the painting industry while transitioning from manual production methods to the development of machine tools and factory systems.Reference Hong, Candelone, Patterson and Boutron1,Reference Nriagu42 Despite the risk associated with white lead, (extensively reported in medieval texts) such as apoplexy, epilepsy, and paralysis, the pigment became extremely popular for its desirable density and opacity. Being washable and extremely durable, the uses of lead-based paints evolved covering virtually all imaginable surfaces ranging from industrial to domestic applications, thereby increasing the pathways of lead exposure. Among domestic applications, it even became common to use leaded paints for children’s toys.Reference Needleman66 Remarkably, it was not until 1892 that the Australian physician Turner (1861–1947) discovered the adverse effects of lead on children. He discovered that many children previously diagnosed with meningitis instead suffered from plumbism, which was traceable to the use of leaded paint on items in the children’s homes.Reference Turner67 Alongside with efforts from ophthalmologist Gibson (1860–1944), who diagnosed retinitis and ophthalmoplegia in lead-injured children, lead was banned from domestic paint in Australia in 1914—the same year childhood lead poisoning was only first reported in the United States.Reference Gibson68,Reference Needleman69 Taking into account the pica-behaviour, which is the tendency to crave eating non-food items among children ranging from one to two years, one can understand the large impact of lead-painted toys on childhood lead poisoning.Reference Glickman, Chaudry, Costantino, Clack, Cypess and Winslow70 In like manner, lead-contaminated dust from leadlights and deteriorating indoor and outdoor leaded paints presented an additional pathway of lead intoxication in domestic environments, both for children and adults.Reference Sayre, Charney, Vostal and Pless71 Strikingly, in 1923, the National Lead Company (NLC) hired the fledgling advertising industry to persuade the consumer that lead was child friendly.Reference Markowitz and Rosner72,Reference Nye73 A booklet named The Dutch boy’s lead party was sent to thousands of paint stores containing the cheerful verse “A little toy lead soldier once to the Dutch Boy said, ‘We have some fine relations who all contain some lead’. Why don’t you give a party so folks can see the other happy members of the great lead family?”. A few years later, the NLC distributed The Dutch boy’s Hobby: A paint book for girls and boys.Reference Markowitz and Rosner74 The famous trademark character appeared on the cover, brandishing a ladder and a loaded paintbrush, mounted upon a strange pony whose body was a lead ingot and whose head was a bucket of paint. Only by the early 1940’s, it was demonstrated that plumbism resulted in permanent impairment in children, and it took nearly two more decades to recognise that even silent doses of lead cause severe deficits.29,Reference Needleman69 At that time, the first regulations began to take shape in which primary precautionary measures were taken to protect human health from lead exposure. In America, mandatory testing programmes were established to detect and identify early stages of poisoning. Remarkably, although lead-based household paint was banned in Australia already in 1914 and by international convention in 1925, the United States was not a signatory to the latter agreement, only banning leaded paints by 1970.Reference Lin-Fu75 As a result of the high risk factor associated with infants, in 1991, the Center for Disease Control and Prevention (CDC) devised a strategic plan to prevent childhood lead poisoning.76
Furthermore, occupational health was gradually being acknowledged as an important governmental public health issue.Reference Staudinger and Roth77 In the early 20th century, the United States and several European countries enacted legislations designed to protect industrial workers from potential dangerous toxic environments.Reference Gidlow78 The worst outbreaks of adult lead poisoning of that time were of occupational origin and it had become public knowledge that working in an industry handling lead was certain to make you sick. Workers absorbed lead from inhalation of fine dust or fumes, contamination of food eaten at the workplace, or by absorption through skin. Dickens (1812–1870) described in his essay Star of the East the horrible effects of lead poisoning on a woman who worked in London’s infamous white lead mills as “her brain is coming out her ear and it hurts her dreadful”.Reference Needleman51,Reference Warren58 At the turn of the 20th century, British factory inspectors even noted the hazards to the reproductive system and described women, who were exposed to lead through working in cottage ceramics, likely to be barren or have short-lived children.Reference Lessler59 The United States Congress recognised the severity of the problem and passed the Occupational Health Act.Reference Gidlow78 This legislation created the National Institutes of Occupational Safety and Health (NIOSH) that ever since are responsible for conducting research and making recommendations for prevention of work-related illnesses.Reference Landrigan and Todd79
Contemporary history (1945–Anno 2017)
With increasing evidence of lead poisoning accumulating at that time, extended protection of the public became more evident. Primordially environmental lead accumulation was recognised as the culprit of lead poisoning. System analysis studies were used to study the transfer of lead from the workplace, into the environment, and from thereon to the population.Reference Lessler59 Besides the detection of lead in the every-day environment, it became possible to identify the pathways of lead intoxication in society and devise ways to significantly reduce its accumulation and emission. As indicated in box F, perhaps the most burdening compound of lead in the 20th century is the octane-boosting gasoline additive tetraethyllead (TEL).Reference Nriagu41,Reference Needleman69 Discovered by Midgley, Jr. (1889–1944) and Kettering (1878–1958), founder of Delco and head of research at General Motors, TEL shifted the gears of lead production in the early 1920s.Reference Hong, Candelone, Patterson and Boutron1 TEL was raved for its quality to allow engine compression to rise substantially, which in turn increased vehicle performance and enhanced fuel economy. Contrarily, this poisonous gas required to be handled like a chemical weapon that also had been considered by the United States war department.80–Reference Edgar82 The manufacturers calculated that they could sell an approximate 60 million tons of TEL per year.Reference Hall83 Remarkably, at that time, ethanol was widely known as an inexpensive, low toxicity octane booster. Despite its availability, the manufacturers, who benefited from a uniquely profitable patent, promoted the lead-based anti-knocking agent as the oil industry remained opposed to the alcohol octane booster.Reference M84 Sold by Ethyl Corporations, founded by General Motors and Standard Oil of New Jersey (Currently Esso), the detrimental effects of TEL have brought publicity upon themselves through the poison victims at the Standard Oil plant at Bayway, New Jersey.Reference Landrigan85 With a reported number of deaths, it was noted that lead poisoning resulted in acute mania among a large number of workers, leaving TEL named as the Insanity gas of Bayway.Reference Kovarik86
As a result, Kettering hired Kehoe (1893–1992), an American toxicologist and leader in occupational health, to develop protocols for the workers handling TEL. Kehoe soon became the foremost medical apologist for the use of TEL in gasoline, advocating its safety while gaining prominence as the industry expert at government and public health hearings.Reference Kehoe87–Reference Kehoe, Cholak, Hubbard, Bambach and McNary89 As almost all research funds concerning leaded gasoline came from the industry and were mostly channelled via him, Kehoe held complete monopoly on data for nearly half a century, thereby using the authority of science to cloak the threat to the public health and the environment.Reference Nriagu41,Reference Needleman90 Claiming that lead was naturally present in humans and other organisms, he argued that exposure to low levels of lead were harmless.Reference Kehoe, Cholak, Hubbard, Bambach and McNary89,Reference Kehoe91 His misconception on ‘natural’ lead levels remains existent to present-day society and is often a source for the misguided advocacy on the use of lead-based compounds, as has been the case for lead halide perovskites on multiple occasions.Reference Park, Grätzel, Miyasaka, Zhu and Emery92 Although lead toxicity is known since antiquity, it was not until numerous scientific studies conducted by Patterson (1922–1995), and above all his findings in Contaminated and Natural lead Environments of Man published in 1965, that our perceptions on lead in the environment were forever challenged.Reference Patterson, Ericson, Manea-Krichten and Shirahata26,Reference Patterson93–Reference Murozumi, Chow and Patterson95 Patterson, who was initially funded by the oil industry, argued that lead contamination was taking place with a clear onset since the start of Industrial Revolution and markedly accelerated since the introduction of leaded gasoline.Reference Needleman90 Encountering strong opposition from those then considered experts, such as Kehoe, he endured his efforts against the lobbying of the oil industry, finally ensuring lead being removed from gasoline.Reference Lovei96 Through ice-core samples taken in Greenland in 1964 and Antarctica in 1965, he was able to demonstrate the rise of atmospheric lead levels throughout human history due to anthropogenic sources.Reference Hong, Candelone, Patterson and Boutron1,Reference Murozumi, Chow and Patterson95 Furthermore, he heavily objected against what Kehoe would describe as ‘natural’ lead levels being rather ‘typical’ lead levels, emphasizing that even if certain levels were commonplace it did not mean they were harmless. ‘Natural’, he insisted, was limited to concentrations of lead that existed in the body or environment before anthropogenic contamination.Reference Patterson93 Following Patterson’s criticism of the lead industry, he was refused contracts with many research organisations, including the supposedly neutral United States Public Health Service.Reference Tilton97 In 1971, he was excluded from a National Research Council (NRC) panel on atmospheric lead contamination, even though he was the foremost expert on the subject.Reference Tilton97
In spite of that, the United States at long last mandated the use of unleaded gasoline in all new cars manufactured from the 1975 year model. However, the phase-out was initially not intended based on environmental and health concerns, but rather to protect the catalytic converters that in the presence of TEL could not adequately convert the polluted gas from the internal combustion engine into less harmful exhaust gasses.Reference Gidlow78 Only later, the detrimental effects of lead were acknowledged and by the early 2000s the phase-out was completed in most industrialised countries. In parts of Algeria, Iraq, Yemen, Myanmar, North Korea, and Afghanistan, it remained legal until late 2014. As of 2016, the United Nations Environment Program sponsored phase-out of leaded gasoline is nearly complete, except for Algeria, Yemen, and Iraq.Reference Tsai and Hatfield98 The severe reduction of lead in gasoline, controlling the use of lead pigments in paint, and banning lead-based glazes on pottery and ceramic ware have resulted in a reduction of industrial as well as population exposure to lead.Reference Astrid Sigel, Roland and Sigel99 Average blood lead levels for both children and adults have dropped more than 80% compared to the references values of the late 1970s.Reference Lessler59,100
Lead legislation
In this section, general information on the legislations concerning the exploitation of lead (and to some extent cadmium) in consumer products is discussed on a global level. With the swift surge forward during the technological revolution of the past century, and our continuously advancing knowledge on the hazardous effects of substances used in customary devices, the enforcement of corresponding legislation became of extreme relevance at the start of the 21st century.
Europe
In the present fashion-conscious society, in which newer technologies arrive at an ever-increasing rate, consumers discard their obsolete products sooner than ever, causing the accumulation of enormous amounts of hazardous waste persistently piling up on landfills.Reference Leung, Duzgoren-Aydin, Cheung and Wong101,Reference Belevi and Baccini102 As such, refuse of electrical and electronic goods represents the fastest-growing waste stream in the European Union (EU), with some 9 million tons generated in 2005 and an even higher 12 million tons estimated by 2020.35 The Restriction of Hazardous Substances (RoHS) directive, enacted by the EU, is set up to address the global issue of hazardous consumer electrical and electronic wastes by restricting the use of hazardous materials in electrical and electronic equipment (EEE).34,103 Following the Waste Electrical and Electronic Equipment (WEEE) directive (2002/95/EC) concurrently enacted in 2003 that sets collection, recycling, and recovery targets for electrical goods the ultimate pursuit is to improve environmental management with the additional economic benefit of recovering scarce and costly resources (e.g., approximately 10% of the worldwide gold supplies).35 Besides the high-tech trash problem, the RoHS directive does not seek to solely address acute toxicity of hazardous compounds. As present-day technologies enable the detection of minute quantities of environmental intoxicants, the severe consequences of neurological, developmental, and reproductive impairments induced upon chronic contact with trace amounts have been much more comprehensively understood. Therefore, unlike former regulations and legislation, the RoHS directive furthermore reflects on research in biological and environmental toxicology of the past century, distinctively acknowledging the long-term effects of low-level chemical exposure on the population.Reference Groß, Bunke, Gensch, Zangl and Manhart104
RoHS 1 (2002/95/EC) was adopted in February 2003 and took effect in July 2006, becoming a law in each EU member state, each using their own enforcements and implementation policies with the directive as a guideline.103 The directive initially restricted the use of six hazardous materials (directive 2015/863 adds four additional substances) in various types of EEE; relevant to this work are the restriction of the heavy metals cadmium and lead. For the latter reason also referred to as the “lead-free” directive, the RoHS 1 only allows a maximum permitted concentration of 0.1 wt% (or 1000 ppm) in non-exempt products, except for cadmium for which the limit is set at 0.01 wt% (or 100 ppm). However, crucial to the interpretation is that the limits do not apply to the weight of the finished product, or even to a component, but on each individual homogeneous material in the product that could (theoretically) be separated mechanically—e.g., the sheath on a cable or the tinning on a component. In other words, materials that qualify as a homogenous material must meet the maximum permitted limit.
In an effort to strengthen the RoHS 1, the European Commission (EC) reviewed thus far excluded product categories to cover additional EEE. Improving regulatory conditions and legal clarity, RoHS 2 (2011/65/EU) evolved and was enacted in July 2011, taking effect from January 2013.34 Perhaps the most crucial amendment was the newly imposed higher level of strictness in demonstrating conformity with the directive. Not being able to show rigorous compliance in sufficiently detailed files and not ensuring RoHS 2 implementation in the products were from here on considered as a lack of diligence prosecutable as criminal offense. Remarkably, for the benefit of technical and scientific progress, the RoHS does allow the request for limited exemptions—by substance, category, substance location, or weight—provided that such inclusion does not weaken the environmental and health protection. Notably, novel research allows companies to release state-of-the-art RoHS conforming products that are currently exempt from compliance, e.g., the lead-free packaging technology announced by IBM as a solution for high lead solder joints.Reference Shih, Dang, Gruber, Lu, Kang, Buchwalter, Knickerbocker, Perfecto, Garant and Knickerbocker105 Currently, a few tens of exemptions exist with a lifetime ranging from five to seven years. Since RoHS 2 taking effect, exemptions for which no application of renewal is submitted in due time will expire on the date specified in the RoHS article 5 or in RoHS annexes. As a consequence of the above, the realisation of RoHS conforming products requires comprehensive knowledge on the exempted use of each restricted compound. In order for the manufacturer to declare that their product meets all the legal requirements in scope with RoHS 2, the product must display the Conformité Européenne (CE) marking along with the manufacturers’ name, address, and a serial or batch number. The CE marking signifies that the product may be sold in the European Economic Area (EEA) without any restrictions and has been assessed to meet high safety, health, and environmental protection requirements.106 Additionally, the marking supports fair competition by holding all companies accountable to the same rules.
China, Japan, and the United States
Manufacturers outside of the EU must also comply with the RoHS 2 directive, and thus CE marking, if they wish to import their products to the EEA. This can, however, be rather challenging as foreign regions have their own regulations and legislations. As the world’s leading manufacturer, the Chinese government has their own legislation to control certain materials, including lead.107,108 Entitled Administration Measure on the Control of Pollution Caused by Electronic Information Products, or more commonly known as the Chinese RoHS, the Chinese government has established similar hazardous substance restrictions following a different approach.Reference Kuschnik109 Unlike the EU RoHS 2, where certain amounts of materials in products are prohibited unless exempted, the Chinese RoHS 1 consists of a list of included products (referred to as the catalog available both in Chinese and English) to which the regulations apply.Reference Yang110 In July 2016, the Chinese RoHS 2 came into force, expanding the catalog subjected to mandatory compliance with hazardous substance restriction limits.Reference Zhu, Geng and Sarkis111 Non-listed products in the catalog that contain certain hazardous substances exceeding the Chinese RoHS 2 limits can still be sold in China. However, in analogy to the CE marking, the latter products and those that fall under the covered scope of the Chinese RoHS 2 must be provided with markings and disclosure as to the presence of a certain substance.
Japan, representing the world’s largest consumer electronics industry, has no direct legislation dealing with EU RoHS substances.Reference Shin112 However, its recycling laws (Effective Utilisation of Resources) have spurred Japanese manufacturers to move to lead-free processes in accordance with the RoHS 2 directive (except for phthalates). Effective since July 2006, the Japanese industry standard JIS C 0950:2005, entitled Marking of Specific Chemical Substances (more commonly known by the acronym J-MOSS), additionally enforces the labeling of six specific chemical substances (including lead) for seven specific customary electrical and electronic product categories (personal computers, unit air conditioners, TVs, electrical refrigerators, electric washing machines, microwave ovens, and cloth dryers).Reference Aizawa, Yoshida and Sakai113 In January 2008, JIS C 0950 revised its marking system, though this is out of scope of the current work.
Within the United States, ranked as the world’s third largest economy in 2016, the restriction of hazardous substances is covered within the Californian Electronic Waste Recycling Act (EWRA), passed in 2003.114 This act prohibits the sale of electronic devices starting from January 2007 in compliance to four restricted heavy metals of the EU RoHS directive. It must be noted that the EWRA directive is restrictive across a much narrower scope, applying to CRT, LCD, and plasma display devices contained in televisions, computers, and EEE with a screen size over 10 cm in diagonal. The act also requires retailers to collect an EWRA fee, effective since January 2005, from consumers purchasing covered devices. Electronic devices containing toxic metals and not complying with the act may not be manufactured, sold, or imported into California. Moreover, currently 27 other member states have either effective or pending regulations modeled after the EU RoHS directive. In effect since January 2010, the California Lighting Efficiency and Toxics Reduction Act applies the EU RoHS restrictions to general lighting.115
Worldwide industry standards
Apart from the regulations and legislation above, there also exist other environmental standards established by companies. Manufacturers will find it cheaper to have a single bill of materials (i.e., the product structure) for a product that is distributed worldwide, instead of customizing the product to fit each country’s specific environmental laws. To achieve such standardised processes, companies often develop their own standards by allowing only those substances that fall under the strictest collective official restrictions worldwide. A near epitome is the Product Content Declaration enforced by IBM to its suppliers to conform to the IBM Baseline Environmental Requirements for Materials, Parts, and Products for IBM Logo Hardware Products.116,117 As an illustration, IBM banned decaBDE from its manufacturing processes even though the compound was exempted from RoHS 2 (overturned by the EC in 2008).118 Another representative example is the similar environmental standards set by Hewlett–Packard.119
Criticism of the RoHS
Despite the constructive nature of the RoHS objectives, the directive received several negative criticisms during its implementation. The first was the result of its seemingly adverse effects on product reliability and quality due to the compliant use of (expensive) lead-free solders, which has caused considerable commotion in the soldering industries—especially in smaller businesses that suffered most from the costly enactment.120–Reference Puttlitz and Stalter122 The long-term reliability was of particular concern due to the growth of tin whiskers associated with the use of (lead-free) tin solders (to a smaller and slower extent also observable in lead-tin solders).Reference Galyon123–Reference Vianco and Frear125 Meanwhile, there are a variety of approaches used by manufacturers to mitigate the whisker issue, such as tin-zinc formulations that produce non-conducting whiskers or formulations that reduce whisker growth.Reference McCormack, Jin, Chen and Machusak126,Reference Abtew and Selvaduray127 As of 2013, millions of compliant products are in use worldwide, and it must be noted that so far no significant number of whisker-failures has been reported. The only recorded example with marked economic effects was the 5% failure rate in certain components of the Swiss Swatch watches that triggered a US$1 billion recall in 2006.128
A second criticism on RoHS 2, perhaps most relevant to this work, is that the restriction of lead does not address some of its most prolific applications. More specifically, the strictly and costly regulated use of lead in EEE only accounts for less than 10% of the world lead consumption, whereas 80% of lead is used in lead-acid batteries that are covered by the Battery (2006/66/EC) directive—that does not restrict the use of lead (but does restrict cadmium and mercury).129–Reference Pavlov131 A similar criticism is that less than 4% of lead in landfills is due to EEE, whereas 36% is due to leaded glass in cathode ray tube monitors and televisions that contain up to 2 kg of lead per screen.Reference Herat132 Not entirely in scope of this work, but important to note, is that as a result of the above, the effectiveness of the RoHS directive can be challenged in the sense that it only governs restricted use of the lead contributing least to the environmental accumulation. Albeit the Battery directive enforces the recycling of lead as far as technically feasible with a recycling minimum of 65% for lead-acid batteries, the hazards associated with the liberation of harmful lead compounds upon worst-case scenarios are not as tightly regulated and covered as is the case with the RoHS 2 directive.130 To put the latter into perspective, consider that 60% of an automotive-type lead-acid battery rated at 60 A h accounts for approximately 8.7 kg of lead. In a fully charged state, the negative plate consists of metallic lead and the positive plate of lead dioxide (PbO2), whereas in the discharged state both plates consist of lead(II) sulfate (PbSO4).Reference Pavlov131 Lead(II) sulfate, being corrosive, is known to be extremely harmful by inhalation, ingestion, and dermal contact. Furthermore taking into account that lead dioxide is an intermediate product in lead smelting and considering the reactivity of the metallic lead anode upon contact with the acidic electrolyte (H2SO4) within the battery, it is conceivable that similar to soldering, increased temperatures in a vehicle fire can readily liberate substantial amounts of adverse lead fumes to the nearby surrounding. Assuming that only 10% of the battery would react in the fire, an astonishing 17.8 tons of lead can potentially be liberated based on the 204 500 vehicle fires reported in the United States in 2015.133 As a vehicle fire represents one of the most common causes of fire-related property damage, it can thereby impose an immediate burden on human health in the form of adverse lead fumes. By that, the numbers above disprove the criticism of the RoHS 2 directive with respect to its sole application to EEE and leave room for discussion concerning the protection offered by the current Battery directive.
The case for lead halide perovskites
Historical reflection
The historical exploitation of lead clearly connotes that it has been a chief metal in human history, being among the first metals discovered and used by prehistoric man. Finding purpose in a wide array of applications, ranging from industrial employment to domestic, medicinal, scientific, and artistic use, it is noteworthy that lead is a knife blade that cuts both ways.
Despite well-documented records of respected and established figures on the detrimental effects of lead poisoning throughout history, it is puzzling to see plumbism persisted time and again, resulting in the most widespread anthropogenic pandemic in history. Having invented many tools, equipment, and concepts that are still used today, and thereby having laid the fundamental building blocks of science, literature, arts, architecture, and finances that humanity has capitalised on to date, it is bewildering that the highly-evolved classical civilisations did not deny lead from their society. As illustrated in Fig. 1, there is a surprisingly clear match between fluctuations in lead mining and applications associated with each time period that resulted in mass lead poisoning. In spite of nearly four millennia of oscillations in lead consumption, history repeated itself on multiple occasions and lead blindness kept growing—even far after the first edict ever banning its use was issued. As public and occupational health only started to become a governmental issue at the beginning of the 20th century, legal prohibitions of the heavy metal until that time were regrettably not based on environmental or health concern, but were rather motivated by economic factors. However, it cannot be denied that the sequence of these events has shaped our present-day legislatorial system on lead exploitation, and while it appears that the detrimental effects of lead are now fully recognised and more comprehensively understood, it likewise cannot be ignored that the commercialization of lead halide perovskites can anew form a burdening pathway of lead into society. This becomes particularly alarming considering that already on multiple occasions in the context of photovoltaic perovskites ‘typical’ lead levels are referred to as ‘natural’—while exactly the opposite has been proven to be true in 1965.Reference Park, Grätzel, Miyasaka, Zhu and Emery92,Reference Patterson93 The historical overview (vide supra) aptly details how the attractive and diverse properties of lead have caused its use being condoned in food, non-food, and highly technological applications, with some of these offering more direct contact with the consumer as compared to others. Therefore, realistically, and depending on the application, it must be considered the same can as well hold true for the commercial exploitation of lead halide perovskites. Considering the present-day knowledge and above all experience on the hazardous effects of lead, it is highly appropriate to critically use this awareness to scrutinise and question if the commercial large-scale implementation of lead halide perovskites under current conditions can inadvertently cause an unfortunate repetition of history.
Ambiguity in law by definition
To determine whether lead halide perovskites for photovoltaic applications in general comply with the RoHS 2 directive, it becomes essential to define the device architecture (i.e., stacked layers) that will be used in the resulting product. As indicated above, the RoHS 2 allows 0.1% lead in non-exempt homogenous materials, and depending on the device architecture, either planar or a mesoporous, the definition of a homogenous material as reported in the RoHS 2 becomes inadequate and ambiguous. In the case of a planar architecture, in which the device consists of adjacent continuous layers stacked on top of each other, each deposited layer clearly represents a homogenous material as they (theoretically) can be mechanically separated. Bearing in mind that lead accounts for an approximate 33 wt% of the lead halide perovskite, the planar architecture provides a marked offset in the maximum permitted concentration, thereby offering no prospects for compliance with the RoHS 2 directive. On the contrary, in the mesostructured architecture, following the letter of the law, the mesoporous scaffold that is infiltrated with the lead halide perovskite can be considered as a homogenous layer as the two constituents cannot (theoretically) be mechanically separated. In spite of the fact that the mesoporous architecture contains equal or at least comparable amounts of lead as compared to its planar counterpart, the used definition of what constitutes a homogeneous material results in a much more diluted lead content for the mesostructured scaffold. As calculated by Green et al. (2014), if 18 2.5-nm-diameter hemispherical iodide perovskite ‘nanoparticles’ coat a 20-nm-diameter titanium dioxide nanosphere, the scaffold layer will have a porosity of 60% that when additionally infiltrated with Spiro-MeOTAD (having approximately twice the molecular weight of MAPbI3) can reduce the lead content to 0.4–0.5% by weight.Reference Green, Ho-Baillie and Snaith134 Although this calculation is based on an out-dated device design from the early days of photovoltaic perovskites (resulting in poor power conversion efficiency) and in fact still does not provide compliance with the RoHS 2, this example distinctly illustrates how easily any ambiguous interpretation of the directive can give the impression of a significantly reduced (though non-compliant) lead content while remaining the identical environmental burden of lead as compared to its planar counterpart. Nevertheless, overall it can be concluded that despite the ambiguity identified in the RoHS, lead halide perovskite generally do not comply with the RoHS 2 directive.
Ambiguity in law by exclusion
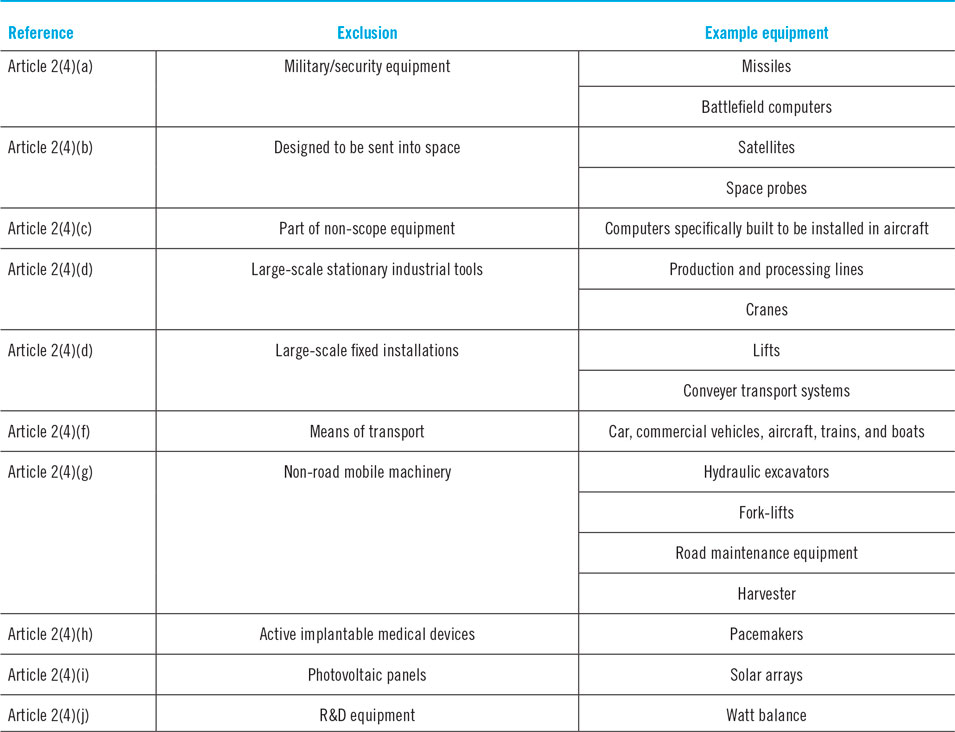
An attainable way to circumvent any compliance with the RoHS 2 directive and its exemptions and remaining rightful to commercialize lead halide perovskite in solar applications, is to exploit the legislation’s so-called exclusions, which are indefinitely effective and apply to a certain selection of technology categories.135 By wrapping the technology such that it qualifies under these current permanently excluded applications as summarised in Table 1, the large-scale commercialization of lead halide perovskites still remains possible. In this respect, the exclusion of professionally installed commercial photovoltaic panels is particularly interesting, as it would serve the main purpose in the commercialization of any photovoltaic technology, including lead halide perovskites. Among other things, it has allowed the commercialization of CdTe photovoltaics, which in scope of the RoHS 2 does not comply either. The European Renewable Energy Federation argued on the inclusion of photovoltaics in the RoHS 2 directive as being a major benefit to the fossil fuel and nuclear energy production, which is far from desirable considering the current need for clean energy generation.136 In excluding solar technologies from the strict regulations of the RoHS 2, the low cost associated with thin film photovoltaic systems could remain ensured, furthermore allowing their growth and, by extension, an increase in the capacity of renewable energies in general (as has been evidenced in the past by the rapid scale-up and production capacities of CdTe photovoltaics). However, according to the customary order of priority in occupational health and environmental protection that strictly command the RoHS 2 and other European directives—being (i) the substitution of hazardous materials should be the top priority; (ii) if substitution is not possible, collective protection measures should be put in place; (iii) ultimately, measures of individual protection apply—the exclusion, though defendable by valid arguments of proponents of the CdTe or lead halide perovskite industry, does simultaneously disregard the raison d’être of the RoHS 2 directive.Reference Saurat and Ritthoff137 Therefore, as long as heavy-metal based applications are allowed by exclusion, there will be heavy metals used in production processes, present in consumer products, and eventually in waste streams; altogether embodying pathways of burden which the directive is designed to limit and prevent. However, if any of the exclusions were to be in scope with the RoHS 2 directive, their employment would require the request of an exemption due to overshooting the maximal permitted concentration of lead. Bearing in mind that exemptions are only deliberated if substitution is impossible due to scientific and technical considerations or if the human and environmental benefits of the substitution are likely to outweigh the negative environmental or health impacts, the RoHS indicates that heavy metal containing photovoltaic technologies can be allowed if (and only if) all alternative PV technologies that do not use any of the prohibited substances cannot replace them. In other words, if photovoltaic panels are ever in scope of the RoHS 2 directive, novel heavy metal containing photovoltaics will not be allowed by exemption as long as substitutive ecological technologies at similar performance level exist—as is currently the case with silicon-based photovoltaics.
Impact resulting from failures during lifetime
The above is particularly relevant when considering the proneness of lead halide perovskite to degrade and potentially leach its lead-based degradation compounds in case of failures. It has been demonstrated that despite great scientific efforts, lead halide perovskites remain prone to degradation upon contact with the circumambient atmosphere, elevated temperatures, and UV-light, from which mainly lead iodide (PbI2) develops as the chief degradation product.Reference Leijtens, Bush, Cheacharoen, Beal, Bowring and McGehee138 As it is only sensible that incidents can occur in each phase of a module’s lifetime—especially common uncontrolled scenarios in the use stage such as hail, (house)fires or whirlwinds—the potential detrimental health and environmental impact of excluding substantial quantities of lead in photovoltaic panels from the RoHS directive become apparent. To put the latter into a more comprehensive perspective, consider a lead halide perovskite absorber layer of a typical thickness of 300 nm containing up to 0.4 g lead per m2.Reference Hailegnaw, Kirmayer, Edri, Hodes and Cahen139 Taking into account that inhalation of toxic fumes presents one of the largest burdens on human health in cases of both organic and inorganic lead compounds, an average roof of 100 m2 that is only covered for 50% with photovoltaic modules can liberate a maximal 20 g of lead per structure fire—excluded the amount of lead liberated from the soldering as will be discussed later.Reference Babayigit, Ethirajan, Muller and Conings18 Moreover, taking into account 763 153 structure fires reported worldwide in 2015 (accounting for nearly 40% of all fire incidents) and furthermore assuming that every future home will be equipped with a photovoltaic installation, an estimated 15 tons of lead can in theory be liberated in one year based on the predetermined conditions above from the absorber layer exclusively.133 Although it has been demonstrated that almost all cadmium from CdTe photovoltaics is encapsulated in the molten glass matrix of the module upon a fire, the latter demonstration is still absent for lead halide perovskites modules.Reference Fthenakis, Fuhrmann, Heiser, Lanzirotti, Fitts and Wang140 However, notwithstanding a similar encapsulation mechanism in rigid lead halide perovskite installations, the numbers above remain of particular relevance when envisioning flexible lead halide perovskite modules. As companies have reported interest in such perovskite modules on numerous occasions, it is important to realize that flexible modules burn more readily as a result of being printed on flexible (plastic) foils and do not provide much encapsulation protection. Furthermore advocating their aesthetic advantages, flexible modules impose considerably more impact as a result of their increased applicability—especially their potential exploitation envisioned in other excluded equipment categories such as means of transport. Bearing in mind that a typical car has a roof surface area of 3 m2, an additional 0.3 tons of lead can be liberated yearly (from the active layer exclusively) in the immediate human surroundings based on the 261 601 vehicle fires reported in 2015 worldwide.133
Impact resulting from solder
On a different note, despite lead-free solders currently being firmly established, the above rationale is also applicable to the vast amount of lead that by exclusion is allowed for the soldering of photovoltaic modules. As the inhalation of lead fumes upon soldering is recognized as a particularly detrimental occupational burden, solder used in contemporary crystalline silicon modules containing up to 35% of lead must also be equally scrutinised.Reference Burge, Harries, O’brien and Pepy141 It has been estimated that 1000 tons of lead have been used for 100 gigaWatts of crystalline silicon solar modules.Reference Werner, Zapf-Gottwick, Koch and Fischer142 Surprisingly, the latter corresponds up to 6 times the lead content in 1 m2 of lead halide perovskite resulting in an overall value ranging between 2.4 and 3 g of lead per m2 in solder.Reference Hailegnaw, Kirmayer, Edri, Hodes and Cahen139 As adverse lead fumes are readily released upon soldering, an additional 91 and 1.9 tons of lead can be released according to the structure fires and vehicle fires reported in 2015, respectively (assuming full evaporation and similar conditions).133
Impact upon recycling at the end-of-life
It has been demonstrated that PbI2, though only sparingly water-soluble (0.76 mg/mL), can produce harmful effects in OECD-recognised model organism zebrafish (Danio rerio) at concentrations as low as 10 µM.Reference Babayigit, Thanh, Ethirajan, Manca, Muller, Boyen and Conings16 The latter is of high significance, as while approaching the application’s end-of-life, lead allowed by exclusion to the RoHS 2 directive can end up in the waste stream. On the consumer side, the stock of heavy metal containing products will keep growing, aggravating the issue of hazardous waste disposal at the end of their lifetime. Photovoltaic systems installed at present-day are expected to last for over 25 years, and it is conceivable that by the time they need to be disposed, the company that produced the modules may no longer exist. The owner of the installation may very well be different from the first buyer, ignorant to any take-back system put in place or reluctant to bear the cost for dismantling his end-of-life photovoltaic system. Therefore, to prevent hazardous substances from ending up in the waste streams, the preferred option should always be to refrain from using such substances. Moreover, for the recycling of lead to be cost-attractive 25 years from now, a high demand for it is needed by that time.Reference Saurat and Ritthoff137 However, as restriction on the use of hazardous heavy metals is only expected to reach further, an increased demand in the future looks unlikely and questionable. The solar industry has launched a voluntary industry initiative, called PV CYCLE, to coordinate the recycling of panels, but legislators have been unsatisfied with its slow progress and its application to solely newly accumulated waste, excluding other historical waste.143 To manage potential future concerns, the EC launched a consultation in 2012 including the regulation of photovoltaic panels under revised rules of the WEEE (2012/19/EU) directive with immediate effect.144 The WEEE mandates European countries to adopt PV waste management programs in which producers are set responsible for the take-back and recycling of the panels they sell. Its goal is on the one hand to encourage industries to develop products that are easier to recycle, while on the other hand it leads producers to factor in the cost of the collection and end-of-life treatment of their products into the cost paid by the consumers. The producers joining these programs aim to contribute to developing greener products, thereby making recycling more affordable and economically sustainable. Currently, depending on the presence of silicon in the photovoltaic panel, thermal or mechanical methods are devised to ensure the recovery of the raw materials. In a recent statement of PVCYCLE, it is announced that present-day recycling rates range up to 90% for silicon based PV modules and up to 97% for non-silicon based modules.145 Thereby exceeding both industry and WEEE standards, many of the recovered materials can be used for supporting the production of new photovoltaic modules and other productions. So far, only lab-scaled recycling initiatives have been demonstrated for lead halide perovskites.Reference Binek, Petrus, Huber, Bristow, Hu, Bein and Docampo146,Reference Kadro, Pellet, Giordano, Ulianov, Müntener, Maier, Grätzel and Hagfeldt147 Nevertheless, as their commercialization is approaching at a very fast rate, strategic recycling plans by its producers are becoming extremely relevant.Reference Kadro and Hagfeldt148
Summary and outlook
Considering the centuries of experience on anthropogenic plumbism and having endured lead blindness time and again since 2000 B.C., it is critical to question if the commercial exploitation of lead halide perovskites will similarly anew provide a pathway of lead into society. Currently, rigorous directives govern the use of lead in consumer products worldwide, and in spite of these legislations being shaped by our prior experiences with the heavy metal, they are not flawless and are still maturing at present-day. Particularly in the framework of lead halide perovskites, apprehensions as well as ambiguity remain in the proper definition of these policies. Moreover, concerns are raised with respect to the excluded applications category that ultimately can allow fairly soluble lead compounds to enter the waste stream upon lead halide perovskite commercialization. Though it is not yet clear that current legislation be ameliorated, it must be noted that unimpeachable directives are imperative to safeguard human health and environment while spurring technological evolutions. Therefore, on the one hand a critical discussion is to be commenced on the regulations and policies governing the exploitation of lead halide perovskites thereby clearly establishing “if” and “how” they can enter the consumer market. On the other hand, being strongly enthusiastic about this revolutionary new solar technology and confident about its large-scale implementation, it is strongly recommended that the periodic poisoning inflicted by lead over the past 4000 years should not be overlooked but rather timely counteracted by intensified R&D efforts.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1557/mre.2017.17.
Acknowledgments
A.B. is a Ph.D. fellow of the Research Foundation Flanders (FWO). B.C. is a postdoctoral fellow of FWO. We thank Dirk Weiss and Andreas Wade for the fruitful discussions.