Perovskites are efficient light emitters and hold promise for making solution-processed, low-cost lasers. Engineers have shown for the first time that continuous-wave lasing is possible with perovskites. In the journal Nature Photonics (doi:10.1038/s41566-017-0047-6), they report coaxing a clear continuous-wave lasing output beam from methylammonium lead iodide films for more than an hour. This is an important first step toward an electrically pumped perovskite laser diode.
Past efforts to get continuous-wave lasing from MAPbI3 perovskites have been unsuccessful. Lasing usually dies down after a few tens of nanoseconds.
Noel C. Giebink of The Pennsylvania State University and his colleagues have found that continuous-wave lasing is possible for the perovskite when it is cooled to below 160 K, the temperature at which the material transitions from its tetragonal phase to an orthorhombic phase.
The engineers speculate that this happens because a sufficiently intense pump generates small tetragonal-phase pockets within the larger bandgap orthorhombic matrix. Light-generated charge carriers accumulate in these energetic pockets, leading to a population inversion—a state in which more atoms in those pockets are in higher, excited states than in lower-energy states—a condition that is essential for lasing. The team says that more work is needed to understand exactly why the mixed-phase material does not experience the lasing death seen in pure tetragonal phase perovskites.
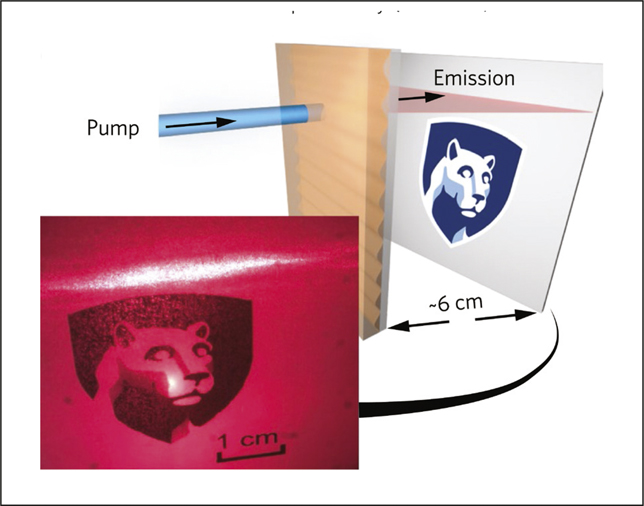
Far-field spatial profile of the laser beam projected onto a white card as depicted in the diagram. The image was recorded by a CMOS camera using a 600-nm long-pass filter to reject stray pump light. Credit: Nature Photonics.
A team of Swiss and US researchers has found the reason behind the extraordinarily fast and bright emission from nanocrystals of cesium lead halide perovskites. The work could have crucial implications for semiconductor quantum dots that are used in displays, lighting, and lasers, leading to materials with much brighter emission.
In quantum dots, light emission is due to excitons: joint states of an electron and a positively charged hole attracted to each other by the electrostatic Coulomb force. In most cases, the excitons from the lowest-energy state, called triplet state, appear as dark excitons due to the slow release of photons from them.
That is not true for CsPbX3 nanocrystals, however, as Michael Becker of IBM Research in Zurich, Switzerland, and Roman Vaxenburg of George Mason University report in the journal Nature (doi:10.1038/nature25147). Lowest-energy triplet state excitons emit brightly in that perovskite’s crystals. This is why light emission happens 20 times faster than in any other quantum dot.
The researchers used group theory and modeling to prove that such bright triplet states exist. They confirmed the existence of the bright excitons by measuring size and composition-dependent fluorescence from single CsPbX3 nanocrystals. Using this approach to identify other semiconductors with bright lowest-state excitons could have important implications, they say.
Researchers at the Weizmann Institute of Science in Israel have unveiled how perovskite single crystals heal themselves.
A team led by Davide Raffaele Ceratti, Gary Hodes, and David Cahen used high-power laser light to create small micrometers-wide damaged spots inside crystals of methylammonium, formamidinium, and cesium lead bromide (MAPbBr3, FAPbBr3, and CsPbBr3).
They measured the photoluminescence of the samples before and after damage. Self-healing occurs in all three materials, they found. Photoluminescence recovered within an hour for MAPbBr3 and FAPbBr3, but took tens of hours for CsPbBr3.
The researchers suggest possible mechanisms for self-healing in their Advanced Materials (doi:10.1002/adma.20170627) paper. The Weizmann researchers show that the self-healing property of these materials could be due to a closed chemical cycle where degradation products such as ABr, PbBr2, ABr3, and Pb(0) can form due to the interaction of APbBr3 with light, which eventually combine in dark and revert to the original perovskite, APbBr3.
The use of lead in perovskite solar cells is a bottleneck to their commercial use. Researchers have put a lot of effort into developing lead-free alternatives. Now, researchers in China report antimony-based perovskite-like materials for solar cells that have efficiencies of over 2%, they write in a paper published in the Journal of the American Chemical Society. The key is to add a substantial amount of chlorine into methylammonium antimony iodide (MA3Sb2I9) perovskites to give high-quality films.
In general, MA3Sb2X9 films are made of either octahedral clusters or a two dimensional (2D) layered structure. The cluster-based structure requires less energy to form, so it is easier to synthesize. The 2D phase, meanwhile, has a bandgap and carrier transport that is more suited for solar cells, but high-quality 2D layered films have been hard to make.
Lijun Zhang at Huazhong University of Science & Technology, Yinhua Zhou of Jilin University, and their colleagues found that increasing the Cl content by adding MACl into the mixture of SbI3 and MAI gives MA3Sb2ClxI9–x perovskites triggers a transformation from the octahedral cluster phase to the 2D phase.
One critical challenge facing today’s perovskite solar cells is the need for hole-transporting materials (HTMs) that are affordable, can be printed using non-toxic solvents, and do not degrade the devices over time.
European researchers have taken an important step toward such market-ready solar cells. They have made a reliable HTM that is easily processed from solution and promises efficient, stable, and low-cost devices. The research team led by Yi Hou and Christoph Brabec of the Friedrich-Alexander-Universität Erlangen-Nürnberg in Germany developed a two-layer hole-extraction contact made of tantalum-doped tungsten oxide and a polythiophene derivative, poly[5,5′-bis(2-butyloctyl)-(2,2′-bithiophene)-4,4′-dicarboxylate-alt-5,5′-2,2′-bithiophene].
They made solar cells by depositing an ultrathin fullerene-phosphonic acid layer as an electron-transporting material and then the hole-transporting bilayer.
The cells had a maximum power efficiency of 21.2%, the highest yet for perovskite cells with ionic dopant-free HTMs, and were stable for more than 1000 hours, the researchers report in the journal Science.


