Metal–organic frameworks (MOFs) are a family of crystalline porous materials consisting of metal clusters coordinated to organic linkers. MOFs are rigid due to their stiff metal-coordination networks. This “stereotype” of MOFs, however, has been overturned by the research group of Matthew J. Rosseinsky at the University of Liverpool. The researchers have developed a “soft” MOF that was shown to adopt nine different configurations. This breakthrough was reported in a recent issue of Nature (doi:10.1038/s41586-018-0820-9).
The key to realizing the soft nature of this novel MOF lies in the design of the linker. The researchers synthesized tripeptides—namely glycine-glycine-L-histidine—as the linkers, and coordinated these to Zn(II) via the deprotonated carboxyl and the imidazole groups of the histidine. Because the chemical bonds associated with the sp 3-hybridized carbon atoms in the tripeptide can rotate, the Zn2+-peptide coordination network (ZnGGH) possesses conformational flexibility. Alexandros Katsoulidis, the first author of the published article, says the design principle of using peptides was inspired by the structural flexibility of enzymes. “These molecules exploit the conformational freedom of the polypeptide chains in order to rearrange their structure according to their environment and perform their function with exquisite specificity,” Katsoulidis says.
ZnGGH readily changes its structure when soaked in different solvents including water, methanol, dimethylformamide (DMF), and dimethylsulfoxide (DMSO). By examining the crystal structures of the solvent-containing ZnGGH by x-ray diffraction, the researchers discovered that the change in the N–Cα bond torsion (ψ) of the central glycine unit of glycine-glycine-L-histidine, a process driven by hydrogen bond formation, was responsible for the conformational flexibility. For example, DMSO formed hydrogen bonds with the terminal amine groups and the glycine-histidine amide groups of the neighboring tripeptides. The hydrogen-bond formation led to a large bond torsion (268°), and resulted in a compact structure with the smallest pore opening (4.5 Å) among all the configurations of ZnGGH.
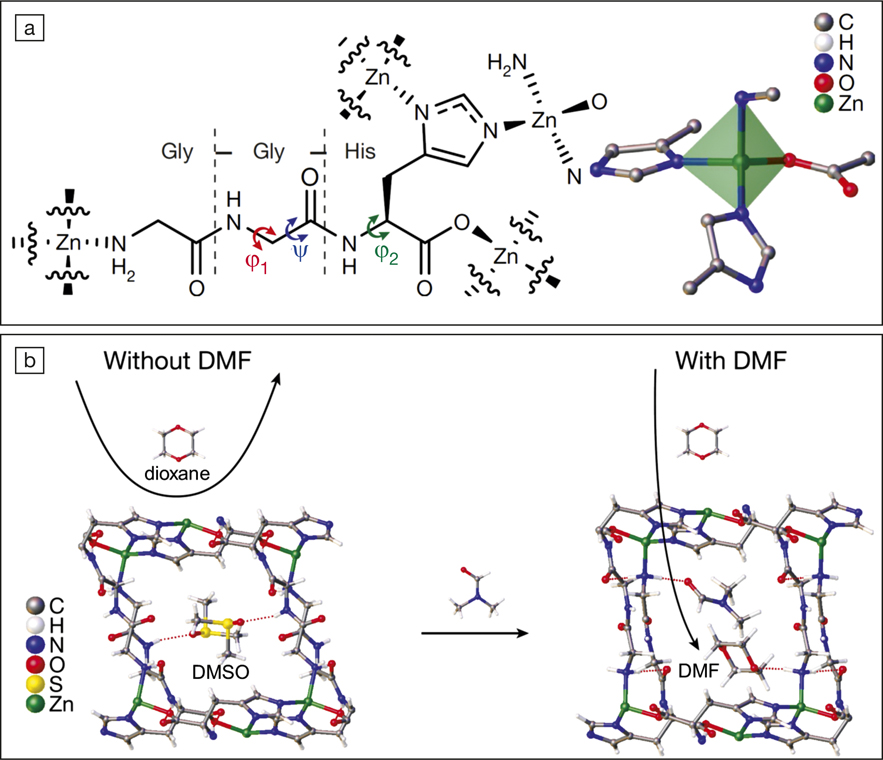
(a) The molecular structure of a Zn2+-peptide coordination network (ZnGGH); φ1, φ2, and ψ are bond torsions that bestow the conformational flexibility of ZnGGH. (b) The exchange of dimethylsulfoxide (DMSO) (left) with dimethylformamide (DMF) (right) enables dioxane adsorption by ZnGGH.
The conformational change rendered ZnGGH capable of hosting various guest molecules. Due to its smallest pores, DMSO-containing ZnGGH was unable to host dioxane—a carcinogenic water pollutant—which was too large to enter the pores. Exchanging DMSO with DMF widened the pores and enabled the adsorption of dioxane.
Scott Oliver of the University of California, Santa Cruz, says that the high level of controllability over MOF conformations is unprecedented, and “the possibilities of using other tripeptides and longer polypeptides for conformation-function control are exciting.” Xinxin Xu of Northeastern University, China, describes this work aesthetically: “The solvent-induced conformational changes of ZnGGH resemble a dancer changing her posture with the change [in] melody.” Both researchers are not involved in the work.
The strategy demonstrated in this work could be extended to synthesize a variety of other structurally flexible MOFs. “We are working toward the formation of flexible MOFs with larger pores and richer chemical functionalities in order to handle larger molecules and perform advanced catalytic and separation processes. Our aim is to achieve precise manipulation of the pore environment, in terms of space and chemical functionality that will enhance the performance of the material on selected applications,” Katsoulidis says.


