Researchers in Holger Frauenrath’s group at the Ecole Polytechnique Fédérale de Lausanne (EPFL) have created carbon nanocapsules varying from 50 nm to 100 nm in diameter through a wet-chemical, UV-assisted carburization technique. R. Szilluweit, T.N. Hoheisel, and colleagues at EPFL and the Swiss Federal Institute of Technology in Zürich fabricated these capsules from amphiphilic hexaynes using a low-temperature carbonization process that allowed them to tune the size of the particles.
As reported in the March 29 online edition of Nano Letters (DOI: 10.1021/nl300822f), the researchers began by synthesizing the amphiphilic hexayne for use as reactive carbon precursors. In a pure state, the hexayne amphiphile was reactive and degraded at room temperature—but the researchers found that diluting the amphiphiles in dioxane/MeOH created a solution that could be kept stable at 4°C for weeks. In aqueous solution, the hexayne amphiphiles experienced a reversible and non-reactive aggregation, resulting in vesicle-like lamellar shells encapsulating a core of water. Passage of the aqueous vesicle solutions through polycarbonate membranes with 50 nm or 100 nm pore sizes and subsequent UV radiation at 1°C resulted in transformation of the self-assembled vesicles into carbon nanocapsules. Small-angle x-ray scattering, transmission electron microscopy, and dynamic light scattering experiments all confirmed they were hollow structures with 4 nm wall thicknesses and radii of 34 nm or 54 nm.
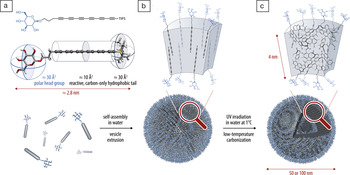
Wet-chemical preparation of carbon nanostructures with controlled morphology and surface chemistry. After self-assembly of the carbon-rich amphiphiles in water and vesicle extrusion, carbonization proceeds under UV irradiation below room temperature in water. Reproduced with permission from Nano Lett. (2012), DOI: 10.1021/nl300822f. ©2012 American Chemical Society.
Although they were prepared at 1°C in water, the nanocapsules have an amorphous graphite carbon microstructure that can typically only be obtained by annealing at temperatures of above 600°C. The structure of the capsules may allow them to be used in high-surface area lithium-ion battery electrodes, while their biofunctional shell of carbohydrates could enable exploitation in drug delivery and cancer detection. The salient discovery, however, has to be that of the process itself, which provides “a new universal strategy for the rational preparation of tailored, functional carbon nanostructures [that] may, hence, open new possibilities for the use of carbon materials in emerging fields of technology.”


