A research team led by M. Saiful Islam of the University of Bath, UK, has investigated how the grain boundaries of solid electrolytes in Li-ion batteries influence Li-ion conduction at the atomic scale. The researchers performed a set of large-scale molecular dynamics simulations that analyzed the Li-ion conductivity at stable grain boundaries of Li3OCl, a promising anti-perovskite solid electrolyte for Li-ion batteries. Their findings were published in a recent issue of the Journal of the American Chemical Society (doi: 10.1021/jacs.7b10593).
Solid electrolytes such as Li3OCl enable the manufacture of all-solid-state batteries with improved duration and safety, mainly due to their nonvolatility and nonflammability. Since electrolytes shuttle Li ions during battery operation, their ion conductivities are of critical importance. “However, the influence of grain boundaries is rarely quantified or characterized in detail especially at the atomic scale,” Islam says. “There is also debate concerning the precise Li-ion migration barriers and ionic conductivity of Li3OCl.” Specifically, the migration barriers estimated by ab initio computational studies are consistently lower than those characterized by experimental work that employs electrochemical impedance spectroscopy.
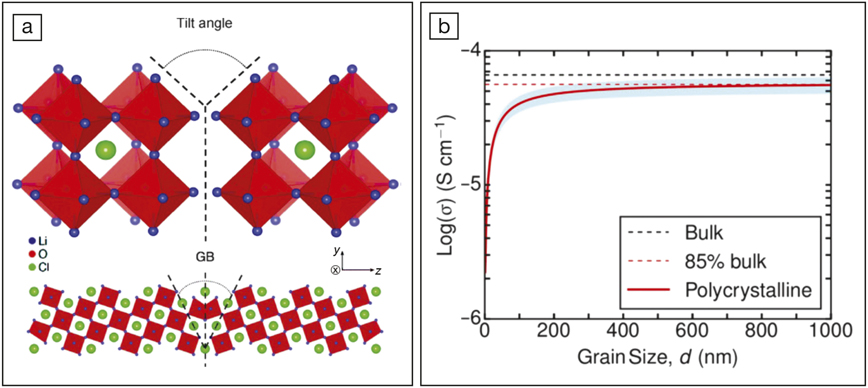
(a) Schematic illustration of a grain boundary (GB) in Li3OCl. A grain boundary is the surface between two crystallites (grains) of different orientations, as represented by the dashed lines. (b) The plot of Li-ion conductivity versus grain size. The red curve represents the simulation results of a polycrystalline Li3OCl at 300 K (where the blue band highlights the upper and lower limits). The black and red dashed lines are the calculated conductivity of a grain-boundary-free bulk material and the maximum average conductivity of a polycrystalline sample, respectively. Reproduced with permission from J. Am. Chem. Soc. 140 (2018), the American Chemical Society.
The researchers attributed this discrepancy to the existence of grain boundaries. They first constructed four typical grain boundaries observed in Li3OCl and evaluated their corresponding energy. The results suggested that the formation of grain boundaries was more favorable in Li3OCl than in perovskite oxides such as SrTiO3. At normal battery operating temperatures (∼0–150°C), all of these grain boundaries exhibited 0.05–0.30 eV higher Li-ion migration energy barriers than the grain-boundary-free bulk counterpart, which suggested that the grain boundaries impeded Li-ion migration. Because previous simulations do not take the grain boundaries into consideration, the simulations over-estimate the true Li-ion conductivity in real Li3OCl samples.
The researchers further developed a polycrystalline model and elucidated the relationship between Li-ion conductivity and the grain size of Li3OCl. This relationship can guide future experimental studies by choosing the optimal conditions for the synthesis of solid electrolytes, as the grain size is closely related to the preparation methods.
Feng Lin of Virginia Polytechnic Institute, who was not involved in the study, says, “For practical polycrystalline solid electrolytes, grain boundaries are complicated due to dynamic evolution of local crystal orientation, defects, and secondary phase impurities. This study provides a nice nucleation point for future elaborate studies considering these practical factors, and will lead to better design of solid-state Li-ion batteries to eliminate the challenges related to ionic conductivity, area specific resistance, lithium dendritic growth, etc.”
“Our approach provides valuable fundamental insights into the role of grain boundaries, which we are extending to other high-performance materials including LISICON and garnet-type solid electrolytes,” Islam says.


