Electric automobiles are becoming more popular and more common on the roads each year. These cars are viable because their battery banks store enough energy to facilitate daily commutes. They also deliver steady day-to-day performance and do not degrade rapidly. However, everyday commutes that are short and intermittent rarely tap the full capacities of these batteries. As long-haul trucks and driverless taxis transition from gasoline to electric power, their constant run time will have an adverse impact on the longevity of their batteries. Furthermore, hot and cold environments also affect the operational capabilities of electric vehicles. There is, therefore, a significant drive to deliver “beyond-lithium” cell chemistries for the next generation of batteries. The aforementioned host of dynamic variables presents a formidable challenge for these efforts: what is the most reliable protocol to evaluate these cells and, over a viable laboratory-scale test process, assess expected lifetime and failure mechanisms that accurately apply to real-life batteries that operate for several years?
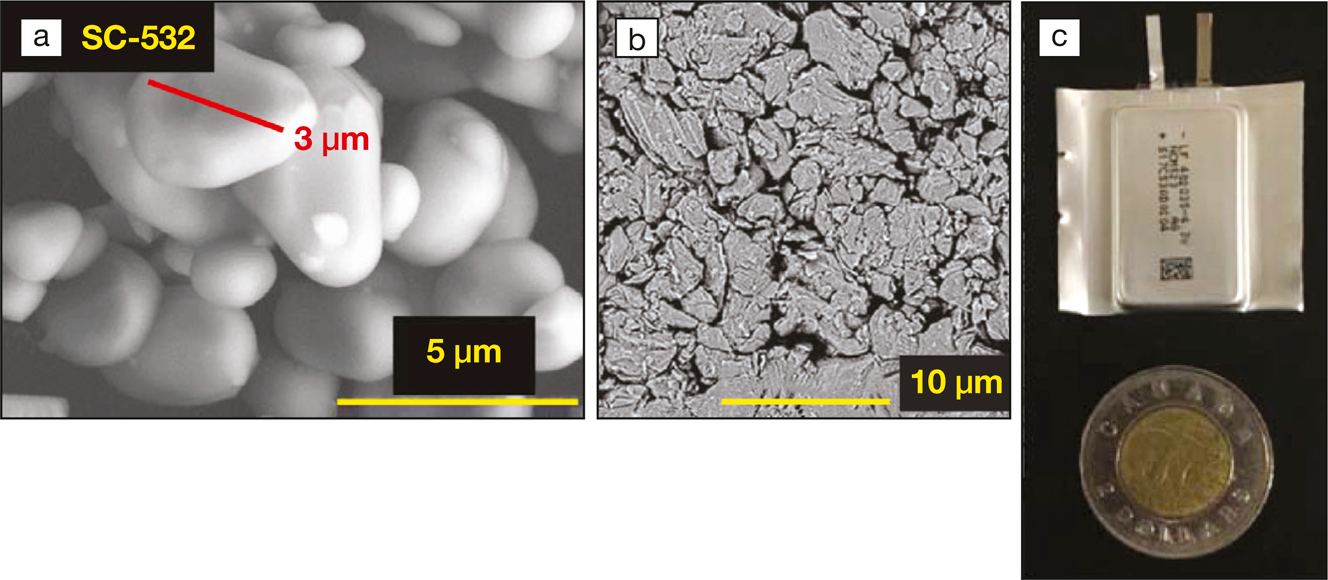
(a) Scanning electron microscope (SEM) image of the single-crystal cathode material; (b) SEM image of the graphite anode material; (c) photograph of a typical pouch cell that was used for this work (coin added for scale). Image Credit: Journal of The Electrochemical Society.
A research group from the Department of Physics and Atmospheric Sciences and Department of Chemistry at Dalhousie University recently reported a lithium-ion cell chemistry design with excellent longevity. Their resulting battery design, which uses finely tuned electrode and electrolyte designs, also withstood temperature extremes, allowed fast charging, and was durable enough to power an electric vehicle for over 1 million miles. The group published their findings and methodology in a recent issue of the Journal of The Electrochemical Society (doi:10.1149/2.0981913jes).
A crucial component of the success of Jeff Dahn and his colleagues was the design of the cathode electrode in the battery cell. A single-crystal electrode composed of a lithium nickel manganese cobalt oxide yielded the highest and most durable capacities. A carefully designed electrolyte that used an organic lithium salt, a blend of three organic solvents, and novel stabilizing agents acted as an essential partner to this electrode and ensured its durability over thousands of charge and discharge cycles. This stable system delivered a 795-Wh/L stack energy density and performed at high efficiencies over 3700 cycles at 20°C and 40°C. The unique design of the cathode ensured that the electrodes never cracked or effused active material.
Georgia Institute of Technology professor Gleb Yushin, who is unaffiliated with this research effort, assessed the impact of the work: “Development of longer lasting batteries [is] critical for both autonomous electric mobility and clean energy grid. Revealing key contributions to cell degradation, as demonstrated by this excellent work and others, enable materials scientists to fine-tune both electrode particles and electrolytes to overcome the current Li-ion limitations. In the near future, I expect new anode and cathode chemistries beyond graphite and NMC [nickel manganese cobalt] will push the limits of energy density versus cycle life to new heights.”
The researchers validated the longevity of their cells against comparable commercial cells and replicated expected operating conditions. The team stored the cells in temperature-controlled ovens for over a year and periodically cycled them to assess their thermal stabilities. Battery testing cycled the energy-storage modules at different rates and combined electrochemistry information with changes in volume (due to aging-induced generation of gas in the cells) and characterization of electrodes at different time points. The researchers meticulously documented their test methods and ensured that researchers could easily replicate these results or build upon them in follow-up efforts. Many of the tested cells are—to this day—still cycling in the Dalhousie University laboratories, and readers are encouraged to contact the research team and inquire about the current operating status.


