Banglin Chen of The University of Texas at San Antonio and co-workers have developed empirical equations to predict the H2-storage capacity of metal–organic frameworks (MOFs), as they reported in a recent issue of Advanced Materials (doi:10.1002/adma.201907995). Their equations illustrate that suitable surface areas and/or pore volumes did lead to high H2-storage capacities.
To derive the empirical equations, the researchers first calculated the pore volumes of six model MOFs for H2 storage based on their crystal structures. Fitting the pore volumes with their experimental H2-s torage capacities revealed a non-linear relationship between the two parameters. Specifically, under 100 bar and at 77 K, the total moles (n total) of H2 stored in cage-type MOFs (MOFs having spherical pores) follow the equation:
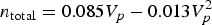 $$n_{\rm total} = 0.085V_p - 0.013V_p^2$$
$$n_{\rm total} = 0.085V_p - 0.013V_p^2$$and those of channel-type MOFs (MOFs having cylindrical pores) fit the equation:
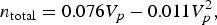 $$n_{\rm total} = 0.076V_p - 0.011V_p^2,$$
$$n_{\rm total} = 0.076V_p - 0.011V_p^2,$$where Vp is pore volume. Both equations indicate that there was an optimal pore volume corresponding to maximal storage capacity. This relationship between n total and Vp was associated with the pore size: Small pores bestow low H2-storage capacity as they offer limited storage space for H2. Large pores decrease the interaction potential between H2 and MOFs, leading to reduced pore filling by H2. Cage-type MOFs stored more H2 than channel-type MOFs at similar pore volumes because the former can interact with H2 via more surface atoms than the latter, which allowed more H2 adsorbed inside pores. The empirical equations predicted that cage-type MOFs with pore volumes around 2.0-4.0 cm3 g–1 would achieve the highest H2-storage capacity.
Guided by the equations, the researchers identified a promising MOF candidate for H2-storage, designated NPF-200. This Zr-containing, cage-type MOF had a pore volume of 2.17 cm3 g–1 and a high volumetric surface area of 2268 m2 cm–3. High-pressure H2 sorption demonstrated that 65.7 mmol H2 was stored 1g of NPF-200 at 77 K and 100 bar. This value only deviated 5.9% from the predicted value (61.8 mmol H2 per gram of NPF-200). More outstandingly, the mass of H2 released from NPF-200 when pressure was decreased from 100 bar to 5 bar at 77 K reached 37.2 g L–1, which was better than that for other state-of-the-art MOFs, and surpassed the goal set by the US Department of Energy (DOE) (30 g L–1 by 2020).
Amanda Morris of Virginia Tech, whose research involves MOFs, says, “The report by Chen et al. expands our knowledge of the design principles for hydrogen storage beyond correlations to the surface area. The empirical equations may enable the design of materials that exhibit capacities at the DOE target, thus, providing a pathway to a widely implementable hydrogen economy.” Morris was not involved in this study.
Addressing future directions of this research, Xin Zhang, the first author of the article, says, “Currently, we are targeting new MOFs with optimal pore properties to reach higher hydrogen storage [capacities]. Additionally, we believe that the methodology used to identify the optimal pore volume for hydrogen storage is applicable to the storage-capacity prediction for other gases under various conditions.”

(a) The predicted total moles of H2 stored versus the pore volumes of metal–organic frameworks (MOFs). (b) Structure of NPF-200. The orange, light yellow, and blue spheres highlight three types of spherical pores in NPF-200. (c) Volumetric working capacity and gravimetric working capacity of NPF-200 at 77 K compared with other MOFs. Credit: Advanced Materials.



