Oxide glasses are disordered molecular structures and typically show poor intrinsic ductility. As a consequence, these materials are brittle. Although it is possible to toughen glasses using additives or heat-treatment methods, their resistance to crack formation and propagation remains very low. To address this long-standing challenge, one strategy is to look at the molecular design of the glass and to tune its composition. By enabling the formation of weak ionic bonds, it has been possible to develop glass compositions that exhibit high crack resistance through the plasticity of their molecular network.
One of these compositions, Cs2O-Al2O3-B2O3, contains Cs+ ions that can form multiple weak ionic bonds with oxygen anions. Under stress, those bonds break and dissipate energy. Also, in the presence of water, new bonds can be formed. Morten M. Smedskjaer, his team and collaborators from Aalborg University and the Technical University of Denmark, and the University of California, Los Angeles, used this rationale to determine this glass composition, and are studying its cracking and healing behavior. Their results were published in Advanced Science (doi:10.1002/advs.201901281).
Although cesium aluminoborate glass has poorer chemical stability, hardness, and strength compared to other glasses with only 2.0 GPa hardness and 20 GPa Young’s modulus, it exhibits a high Poisson’s ratio of 0.32 and an ultrahigh resistance to crack formation (Figure a). The interpretation for these results lies in the ability of the Cs–O bonds to break easily and to induce micro-ductility in the glass network. Microscale shear deformation induced during the indentation experiment could be responsible for preventing crack formation and propagation.
Another unusual feature of cesium aluminoborate glass is its volume recovery under humid conditions over time. The researchers observed that an indentation made under a load of ∼1 N recovered 44% of its volume after only 4 hours at room temperature at ∼40% humidity. Figure b shows another striking example of recovery after indentation at 5 N. This recovery, or healing, is the result of structural changes in the glass network due to hydrolysis. Raman spectroscopy revealed that water molecules diffuse inside the glass and hydrolyze the borates, increasing their reactivity to form new bonds.
This study demonstrates how molecular and compositional designs in glass can be used to create new properties, such as crack resistance and healing. Yet, more work is needed to obtain a more comprehensive understanding of the mechanical deformations at the atomic level. Smedskjaer says, “We wish to investigate further at the medium range order how the glassy network connectivity responds to indentation and hydration. Also, we want to understand the composition dependence of the observed effect of humid aging and if this could be applicable to other more durable glasses.”
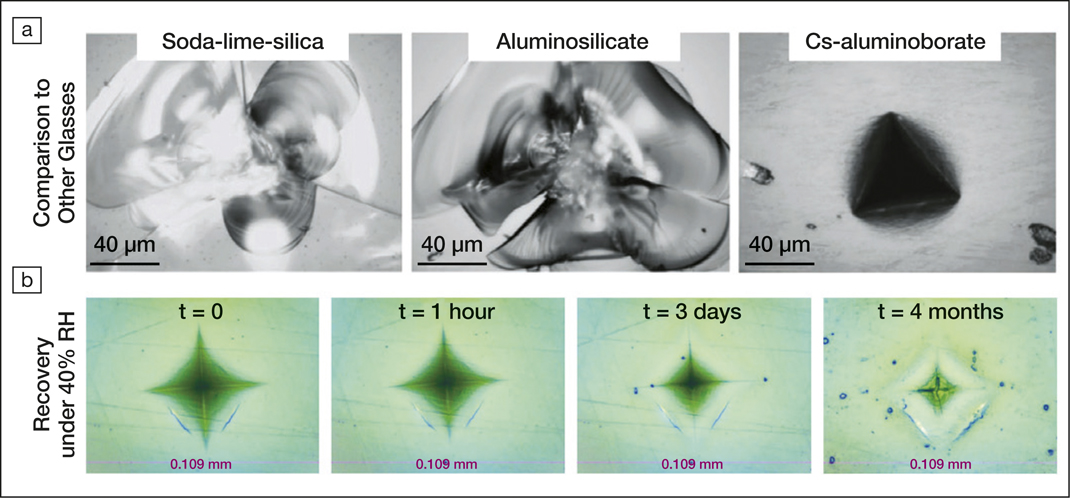
(a) Optical images showing the indentation marks left by a cube corner indenter at 5 N on soda-lime silica, aluminosilicate, and Cs-aluminoborate (this study) glasses. (b) Recovery with time under 40% room temperature relative humidity (RH) of the Cs-aluminoborate glass after Vickers indentation at 5 N. Credit: Advanced Science.
This work on oxide glasses also finds resonance in other fields. Yakai Zhao, a research fellow working on metallic glasses from Nanyang Technological University, Singapore, and who did not take part in the study, says, “The findings of the paper not only present record-breaking crack resistance in oxide glasses, but also enlighten us with new avenues for designing crack-resistant materials through careful design of their chemical composition. It is astonishing to see the excessive volume recovery upon long-term surface aging. I am looking forward to understanding more how to manipulate this environment-assisted recovery to design damage-healable materials.”


