Organ-on-chip platforms are fluidic systems used to monitor the response of a large variety of cell types to drugs and external stimuli in real time. These platforms typically use flat electrodes made of electrically conductive polymers, and the growth and evolution of cell cultures is followed through modulations of the electrical conductivity with time. However, two-dimensional (2D) platforms cannot fully capture the in vivo physiology of three-dimensional (3D) tissues and could result in misleading outcomes. In this context, in a study published in a recent issue of Science Advances (doi:10.1126/sciadv.aat4253), an international collaborative team led by Rόisín Owens from the University of Cambridge reports the development of a 3D tubular strategy to host and monitor growth of cells in 3D.
In this work, a commonly used biocompatible and electrically conductive polymer, PEDOT:PSS, was freeze-dried to create a porous scaffold to support the cells’ invasion. This conducting porous scaffold can be created directly in situ inside a tube or prefabricated, then inserted into a T-shaped tube, called a “tubistor” (see Figure). This represents a 3D transistor that is connected to fluidic tubes to allow a linear flow of the liquid containing the cells, and to three electrodes. The device functions as a transistor and will amplify electrical signals recorded during the experiments. This geometry is particularly interesting, as it can mimic native blood vessels, soft tissues, and even organs.
The researchers tested the device using model mammalian cell lines that were fluorescently labeled. The conductive porous scaffolds were perfused by a continuous flow of cell medium, and the current in the transistor was recorded with time. At the same time, the morphology and number of the cells was monitored by fluorescence microscopy to determine the origin of the changes in electrical conductivity. The researchers observed that after cell seeding, their attachment and growth in the device led to a significant decrease in the conductivity. After two days, the electrical response stabilized, demonstrating that a steady state had been reached. “In the future, we hope to have a system that will monitor longer term effects, such as a week,” says Charalampos Pitsalidis, the first author of the article.
The team plans to use the tubistor for continuous toxicology monitoring. Preliminary data using a drug that bound to calcium have already shown that cells were affected after only 15 minutes. In the future, Owens’s team will perform electrical stimulation experiments with various cell types, including electroactive cells, and study the effect of various compounds on the fully formed tissues.
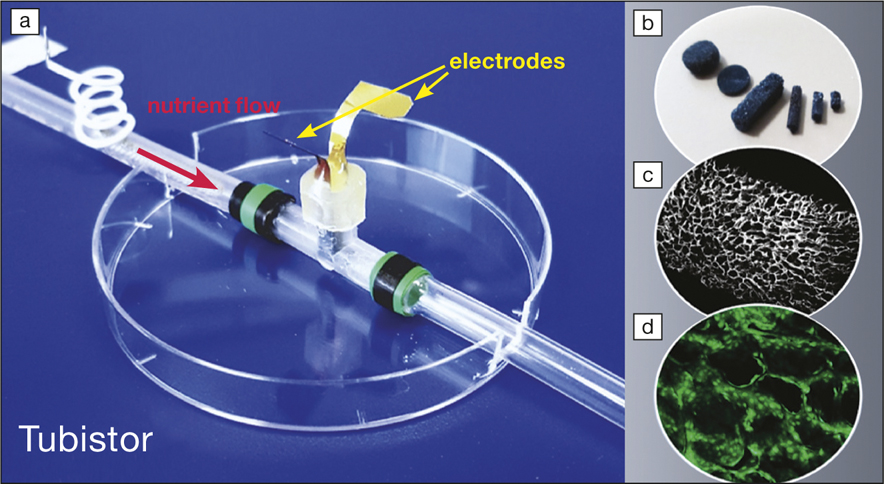
(a) Three-dimensional T-shaped tubistor in an 8-cm-diameter petri dish, with (b) the electrically conductive porous scaffold placed inside, (c) its microstructure, and (d) with green fluorescent cells. Image courtesy of Róisín Owens.
Lesley Chow, assistant professor in bioengineering at Lehigh University and who did not take part in the study, explains that “culturing cells in 3D is necessary to mimic the extracellular microenvironment found in native tissues. The authors demonstrate that advances in bioelectronics can be exploited to help us learn more about how cells respond to their microenvironment in real time. This is likely to have a major impact on tissue engineering, as such 3D devices will accelerate the optimization of biomaterials design.”


