Article contents
COMPARATIVE STUDY BY SOL-GEL ACRYLAMIDE POLYMERIZATION VIA MICROWAVE AND SOLID STATE SYNTHESIS METHODS IN (Er2-x Srx)Ru2O6 SYSTEM
Published online by Cambridge University Press: 30 January 2018
Abstract
We have studied the structural and morphological properties on the pyrochlore (Er2-x Srx)Ru2O6 system, for x = 0.0, 0.02, 0.05, 0.07, 0.10, and 0.15. Polycrystalline samples were prepared by solid-state reaction (SR) and sol-gel acrylamide polymerization (SGAP). Thermogravimetric Analysis (TGA) was used to follow the thermal transformations such as reagents decomposition, phase transformation, chemical stability, and volatilization of organic material of samples. The reagents and synthesized products by the different methods of synthesis were characterized using powder X-ray diffraction (XRD). All samples crystallize Er2Ru2O6 PDF (72-7620) in the cubic unit cell with Fd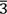 $\bar{3}$m (No. 227) space group and form a solid solution up to x = 0.15. Scanning electron microscopy (SEM) shows considerable variations and similitudes in sizes, very few phases and shapes of polycrystals can be observed. Polycrystalline samples prepared by solid-state reaction (SR) present a grain size varies between 77 nm to 250 nm.
$\bar{3}$m (No. 227) space group and form a solid solution up to x = 0.15. Scanning electron microscopy (SEM) shows considerable variations and similitudes in sizes, very few phases and shapes of polycrystals can be observed. Polycrystalline samples prepared by solid-state reaction (SR) present a grain size varies between 77 nm to 250 nm.
- Type
- Articles
- Information
- MRS Advances , Volume 2 , Issue 62: International Materials Research Congress XXVI , 2017 , pp. 3883 - 3889
- Copyright
- Copyright © Materials Research Society 2018
References
REFERENCES:
- 3
- Cited by


